Separation Conditions3 Separation Using a Column
- Using a Column to Verify That Appropriate Peaks Can Be Obtained
LC-MS Analytical Conditions (Part 2) Flow Injection Analysis discussed how to confirm that target compounds can be analyzed using LC-MS (i.e. that a spectrum of their masses can be detected) by checking that 1) some sort of ions were generated and that 2) the masses of those ions are attributable to target compounds. Part 3 discusses using the column to confirm whether or not appropriate peaks can be obtained and what to do if appropriate peaks cannot be obtained.
Flow Injection-Like Analysis Using a Column
Depending on analytical objectives, chromatography can take a long time for analysis. At the evaluation stage, this time should be minimized as much as possible. In such situations, the evaluation time can be reduced by using a general-purpose column capable of high throughput analysis (such as the Shim-pack FC-ODS, with a 75-mm length and 2.0-mm internal diameter. In other words, target compounds can be eluted more quickly by setting the mobile phase flowrate to 0.4 to 0.6 mL/min, which is 2 to 3 times faster than normal, and increasing the ratio of organic solvent in the mobile phase. (Given A: 0.1 % aqueous formic acid solution and B: acetonitrile, start with ratio of A/B = 1/9.)
In this context, this is called FI-like (flow injection-like) analysis.
There are three points to check for FI-like analysis.
(1) Can the target compound pass through the column?
(2) Do the target compound peaks have a good shape?
(3) Is there any change in the mass spectral pattern of the target compound?
(1) Can the target compound pass through the column?
Since the given conditions result in quick elution for most compounds, the target compound will likely simply pass straight through the column, providing peaks similar to FI analysis.
(2) Do the target compound peaks have a good shape?
The shape of target component peaks can be confirmed at the same time as point (1). Since analysis is based on reversed-phase separation conditions, if peak shape is poor (tailing in particular) with these conditions, then it is easy to predict that satisfactory results will not be obtained under the final conditions that require consideration on the retention of target components and their separation from contaminant components.
(3) Is there any change in the mass spectral pattern of the target compound?
Though a mass spectrum was already obtained using FIA, the spectral pattern can vary due to A) thorough mixing of target components with the mobile phase and because B) passing through the column can reduce the influence of salts in the sample solution.
However, FI-like analysis enables observation of the spectral pattern in the actually-used separation media (column or packing) for chromatography. This is used to confirm whether there are significant differences in spectral patterns (i.e. observed ions are attributable) and what types of ions are advantageous for the mobile phase conditions being used.
Most compounds pass these criteria without a problem, but in extremely rare cases, problems can occur at this step. The following briefly discusses what to do in such cases.
(1) Components do not elute from the column
Some compounds with a strong hydrophobic effect cannot be eluted using an acetonitrile/water (9/1) solution. Given these conditions, substances such as triglycerides and chlorophyll cannot be passed quickly through the column. In that case, a major reconsideration of analytical conditions is necessary.
In other words, 1) change to a mobile phase with a higher elutability, such as acetone or a methanol/isopropanol mixture (assuming separation by reversed-phase mode). Alternatively, 2) consider using a different separation mode (such as normal phase). However, mobile phase solvents generally used for normal-phase separation, such as hexane, benzene, chloroform, and ethyl acetate, are not suited to ionization at atmospheric pressure. Therefore, normal-phase separation cannot be applied to LC-MS by simply switching to a normal-phase column or mobile phase. The key to determining whether normal-phase can be used for LC-MS is whether a mobile phase that allows ionization, such as methanol or acetone (fundamental mobile phases), can be added or not.
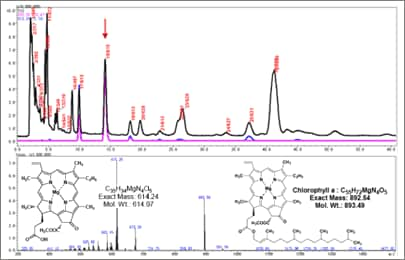
Fig. 1 Example of Analysis Using Normal-Phase Column (hexane/acetone 88:12)
In actuality, there are many examples of using mobile phases such as hexane/acetone(8/2), hexane/ethanol(7/3), or chloroform/methanol(95/5) to perform normal-phase LC-MS analysis (Figure 1). However, with a low proportion of fundamental solvent, these examples also have disadvantages, such as lower sensitivity and stability. Consequently, this solution is selected when the advantages for separation outweigh such disadvantages. Since this involves a major change in mobile phase, it is necessary to start at the beginning and use FIA to reconfirm the ionization method and attribute the observed peaks.
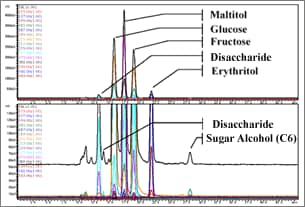
Fig. 2 Sugar Analysis Using Ligand Conversion Mode
LC-MS may be thought as an analytical method with limitations on mobile phases, rather than limitations on columns or separation methods.
Because only water is used as the mobile phase to analyze sugar in the ligand conversion mode (Figure 2), it can be considered suitable for LC-MS analysis.
(2) Peak Shape is Poor
Occasionally, peak tailing is observed during FI-like analysis using a column. If this occurs, try varying the mobile phase pH level slightly. An aqueous formic acid solution is used for these analytical conditions, but no acid was added to mobile phase B, as described in LC-MS Analytical Conditions (Part 1).
Therefore, the proportion of organic solvent was reduced a little and the same analysis was performed to check for any improvement in peak shape. Given the reversed-phase analytical conditions, reducing the organic solvent ratio will increase the retention time, but the acid improves the peak shape for some compounds. If any improvement is observed, good results can be obtained by adding acid to the organic solvent mobile phase as well. This technique applies to analysis for oxolinic acid and new quinolone antibacterial agents (such as sarafloxacin).
(3) Confirming Mass Spectral Patterns
Due to the reasons indicated above, the types of molecular ions observed in FI-like analysis are different than those in FIA. Detecting a variety of ions may be useful for determining molecular masses when identifying unknown compounds (see Part 2), but for quantitative analysis, obtaining fewer types of ions provides higher sensitivity and stability.
In the case of analysis using acetonitrile-aqueous formic acid:
A) If ammonium ion adduct molecules (positive ions) are observed*
Switch the formic acid to ammonium formate to increase the strength of ammonium ion adduct molecules.
B) If chlorine ion adduct molecules (negative ions) are observed*
Add a chlorine solvent to the mobile phase or post-column as a source for supplying chlorine ions.
C) If deprotonated molecules are observed during negative ion analysis
Add ammonium formate rather than formic acid to increase the mobile phase pH and increase the sensitivity for target compounds.
D) If formic acid ion adduct molecules are confirmed
Select good analytical conditions by comparing the intensity of formic acid ion adduct molecules in the fundamental mobile phase with the intensity of deprotonated molecules under non-formic acid conditions. (Do not simply compare ion intensity, but also use S/N for comparison.)
| * | The source of ammonium ions or chlorine ions for ammonium ion adduct molecules and chlorine ion adduct molecules could be from residues in the column or flow lines. |
Fundamental mobile phases used as first-choice mobile phases are based on conditions suited for basic compounds (positive ion analysis). Therefore, targeting acidic compounds tends to require reconsidering more factors. (Mu)
Supplemental Explanation: Based on past analytical experience and other factors, if proper detection of target compounds is expected, sometimes we verify whether samples can be separated using gradient conditions with mobile phases A and B.


