Separation Conditions1 Fundamentals of a First-Choice Mobile Phase
It has become widely recognized that there are a limited number of mobile phases suitable for LC-MS analysis, because compounds must be ionized (i.e. detected). Therefore, even if users do not currently own an LC-MS system, they are increasingly choosing mobile phases that are suitable for LC-MS analysis from the beginning, to allow migrating to LC-MS analysis in the future.
But in reality, many are confused about specifically what kind of mobile phase they should use for LC-MS analysis. Unfortunately, a "dream" mobile phase that can be used for all analytical situations does not exist. In spite of limitation in the types of mobile phases, trying to examine all the various conditions can be a very difficult task. Therefore, to help determine satisfactory analytical conditions in as short a time as possible, we have attempted to create some generalized approaches as a procedure for considering analytical conditions.
Here we will discuss a part of that procedure, regarding the fundamentals of choosing a first-choice mobile phase for two-solvent gradient elution. It's not perfect, but hopefully it will be helpful. So far, using these first-choice mobile phases has provided good analytical results.
Recommended Solvents (fundamental mobile phases)
The electrospray ionization method (ESI) is used for ionizing compounds with relatively-high polarity, by spraying the mobile phase into a strong electric field to form a fine aerosol of charged droplets. Therefore, the following solvents (fundamental mobile phases) are recommended, which must be able to dissolve polar compounds (water and polar organic solvents) and readily form fine charged droplets (solvents with low viscosity and volatile salts).
Recommended Solvents (mobile phases)
- Alcohols, such as methanol or 2-propanol
- Acetonitrile
Acetone - Water or volatile aqueous solutions
Acetic acid or ammonium acetate
Formic acid or ammonium formate
Trifluoroacetate or aqueous ammonia - Volatile ion pair reagents
Perfluorocarbonate (C2 to C8)
Dibutyl ammonium acetate
First-Choice Mobile Phases
Based on the recommended solvents indicated above, try the first-chose mobile phases (mobile phases A and B) shown below first.
First-Choice Mobile Phases
Mobile Phase A: 0.1 % aqueous formic acid solution
• Small molecular weight (M.W. = 46)
• Adjustable to low pH levels
• Low contamination
• Odorless
Mobile Phase B: Acetonitrile
• Ionization efficiency of ESI is higher than methanol
■ Mobile Phase A: 0.1 % aqueous formic acid solution
The reasons for using an aqueous formic acid solution are as follows.
- It has lower molecular weight than acetic acid and trifluoroacetate. In negative ion analysis, these acids are observed as deprotonated molecules and deprotonated dimers, with the advantage being that lower molecular weights cause less interference.
- Added-acid mobile phases are often used for analyzing basic compounds, and LC-MS analysis is often used to analyze pharmaceuticals and other basic compounds.
- In analyses using low-pH mobile phases, it enables keeping residual silanol in an undissociated state, which can inhibit the adsorption (tailing) of basic compounds.
- In terms of preventing contamination of prepared mobile phases, formic acid solvents are generally considered to have lower contamination levels than acetic acid mobile phases. In LC-MS analysis, the purity of mobile phases and additives significantly counts. As evidenced by the commercial availability of LC-MS grade mobile phases, to perform highly sensitive analysis, it is necessary to use particular care regarding the purity of mobile phases and additives.
- Though it is not a scientific issue, formic acid has a less objectionable odor, unlike acetic acid. In many cases, acetic acid is avoided in favor of formic acid because of its odor.
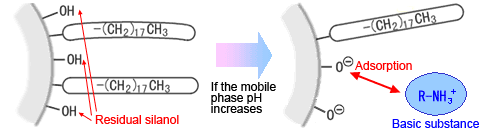
Residual Silanol in ODS Column
■ Mobile Phase B: Acetonitrile
The reasons for using acetonitrile are as follows.
The ionization efficiency of ESI is higher if acetonitrile is used than methanol (lower solvent viscosity is better for producing fine droplets).
Acid is not added to mobile phase B, because it makes it easier to verify the effect of adding acid. In some cases, it is better to add acid even to organic solvents, but ideally, its necessity should be verified before acid is added.
The indicated first-choice analytical conditions are acidic, but for some compounds, it is better to use ammonium salts or basic conditions, rather than acids. Therefore, it is essential that aqueous mobile phases be changed when examining analytical conditions. Changing both an organic solvent and an aqueous mobile phase at the same time will increase the number of types of mobile phases, which will be tricky or troublesome. Further worse, it would be a big waste to let expensive high purity mobile phases gradually lose their purity in storage. Therefore, as a rule, use up LC-MS mobile phases as soon as possible after preparation and take extreme care to avoid contamination when adding substances, weighing, or during other operations.
Column Selection
When selecting a column, preferably choose a sufficiently end-capped column with a silica carrier (3 µm particle size) that has low metal content and chemically-bonded octadecylsilyl groups (such as the Shim-pack FC-ODS, for example). A particle size of 3 µm allows fast separation without requiring application of unduly high pressure, and also allows reducing the time required for examining analytical conditions, such as to verify elution parameters.
LC-MS Analytical Conditions - Part 2, describes performing a simple analysis using the first-choice mobile phase and the process of deciding LC-MS analytical conditions. (Mu)


