Introduction to LC-MS Part2
Part2
On the previous page, we gave an overview of LC-MS analysis and described some of its outstanding advantages, such as (1) it enables obtaining molecular mass and structural information from mass spectra, (2) due to high selectivity, it enables quantitative analysis that avoids the effects of impurities, and (3) it enables analyzing least volatile or thermally unstable compounds. On this page, we will describe the ionization methods used for LC-MS.
Integrating LC and MS
With the success of using GC-MS as a hyphenated analytical instrument, there was increasing anticipation for integrating LC and MS online, but widespread use of LC-MS systems did not begin until relatively recently.
Mass spectrometers remove target components as ions in a gas phase, then detect the ions under high vacuum. In the case of GC-MS systems, target components are already gasified in the GC unit, so they can be introduced directly to the MS unit without further processing. In an LC-MS system, however, if the LC unit is simply connected directly to the MS unit, the liquid mobile phase would vaporize, resulting in large amounts of gas being introduced into the MS unit. This would decrease the vacuum level and prevent the target ions from reaching the detector.
Even eluate of a tiny flowrate of 1 mL/min from the LC unit can expand to 1000 times its volume when vaporized, depending on the type of solvent, which results in a huge amount of gas. Therefore, a key issue for LC-MS is how to get rid of the mobile phase. Consequently, various interfaces were developed, but they all still had problems with sensitivity, stability, user friendliness, or other aspects.
Atmospheric Pressure Ionization Method
When atmospheric pressure ionization (API) appeared not long ago, the improved interface allowed obtaining ions in a reliable manner. As the name suggests, it ionizes samples under atmospheric pressure conditions, which makes it especially useful for removing solvents outside a vacuum. Currently, there are mainly two types of API interfaces. One is electrospray ionization (ESI), which is best suited to ionic compounds with high polarity (Figure 1). ESI draws sample solutions to the tip of a capillary tube, where it applies a high voltage of about 3 to 5 kV. A nebulizer gas flows from outside the capillary to spray the sample. This creates a fine mist of charged droplets with the same polarity as the applied voltage. As these charged particles move, the solvent continues to evaporate, thereby increasing the electric field on the droplet surface. When the mutual repulsive force of the charges exceeds the liquid surface tension, then fission occurs. It is thought that as this evaporation and fission cycle is repeated, the droplets eventually become small enough that the sample ions are liberated into the gas phase (ion evaporation model). ESI provides the softest ionization method available, which means it can be used for highly polar, least volatile, or thermally unstable compounds. Since most of the generated ions are protonated molecules (or deprotonated molecules), complicated fragment ions are not generated. This makes it easy to determine the molecular mass of compounds. In addition, because it generates multivalent ions, depending on the compound, even if a compound has a molecular mass of 10,000, for example, ions with a valence of 20 would only have an m/z ratio of 501 and ions with a valence of 10 would only have an m/z ratio of 1001, which can be detected using a small mass spectrometer. It is also possible to use computer processing to predict the molecular mass from those multivalent ions. ESI is used to analyze a wide range of samples, such as natural substances, biological macromolecules, and pharmaceuticals.
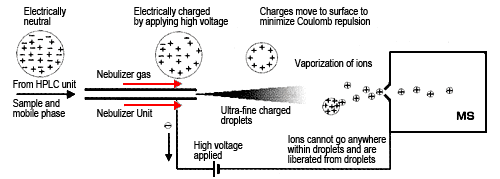
Fig. 1 Evaporation of Ions in Electrospray Ionization (ESI)
The other method is atmospheric pressure chemical ionization (APCI), which is a type of chemical ionization, just like CI for GC-MS (Figure 2). Though the interface design is similar to ESI, the ionization principle differs, making it more suitable for mainly low- and medium-polarity compounds. APCI vaporizes solvent and sample molecules by spraying the sample solution into a heater (heated to about 400 °C) using a gas, such as N2. Solvent molecules are ionized by corona discharge to generate stable reaction ions.
Protons are transferred between these reaction ions and sample molecules (ion-molecule reaction) to ionize sample molecules by either adding or removing a proton. These ion-molecule reactions are known to involve several patterns, such as proton-transfer reactions and electrophilic addition reactions. As with ESI, mainly protonated molecules (or deprotonated molecules) are detected. Consequently, it is used for analyzing highly fat-soluble compounds or compounds that do not ionize in solution.
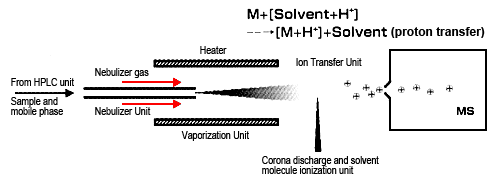
Fig. 2 Ion-Molecule Reactions in Atmospheric Pressure Chemical Ionization (APCI)
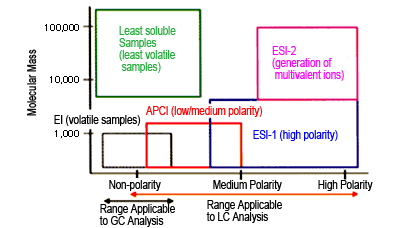
Fig. 3 Ionization Methods and Applicable Compounds
Figure 3 shows the relationship between ionization method and applicable analytes. Since an extremely large variety of compounds can be measured using HPLC alone, selective use of ESI or APCI as the ionization method now allows measurement of wider range of organic compounds as well.


