Synthesis Confirmation for Nucleic Acid Medicines
Medicines utilizing nucleic acids such as DNA and RNA that control genetic information are called "nucleic acid medicines". These nucleic acid medicines allow targeting of molecules such as messenger RNA (mRNA) and micro RNA (miRNA) which cannot be targeted with traditional low-molecular-weight drugs and antibody medicines, and are expected to be innovative next- generation pharmaceuticals for the treatment of genetic disorders which have been difficult to treat so far.
The functions of nucleic acid medicines are various: small interfering RNA (siRNA) to control protein synthesis by coupling with mRNA, miRNA to strengthen miRNA functions, aptamers that bind to a protein to inhibit its functions, and ribozymes to directly cleave the target RNA are but a few. Their basic structure is a chain comprising a few dozen (deoxy)nucleotides including adenine, thymine, guanine, cytosine, and uracil, which are components of DNA and RNA.
Such nucleic acid medicines can be chemically synthesized without the need to culture cells as in antibody medicines. The obtained molecules are medium-sized having a molecular weight ranging from several thousands to tens of thousands. The confirmation whether or not synthesized nucleic acid medicines are arranged in the intended base sequence is a critical quality characteristic to ensure the action of pharmaceuticals.
This article reports an example of determining a molecular weight and base sequence of synthetic nucleic acid using MALDI-TOF mass spectrometry.
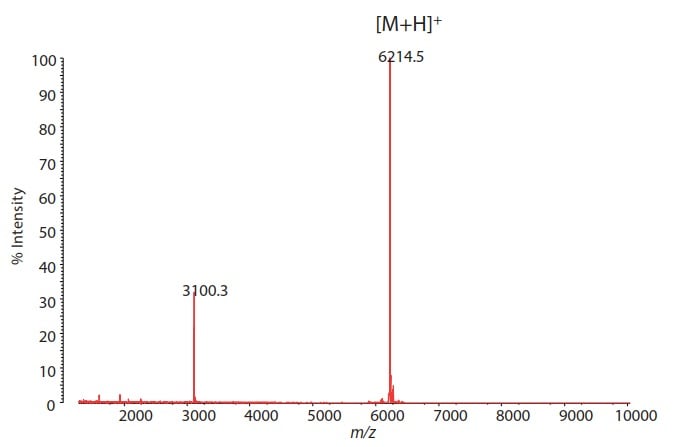
Fig. 1 MALDI-TOF Mass Spectrum of Synthetic Nucleic Acid
Measurement mode: Positive Linear
Benchtop MALDI-TOF MS "MALDI-8020"
Key features:
- Linear mode (positive ion) MALDI-TOF
- 200 Hz solid-state laser, 355 nm
- Load-lock chamber for fast sample introduction
- UV laser-based source cleaning (patented)
- Small footprint/benchtop design
- Quiet operation (<55 dB)


