LabSolutions i-QLinks
Test Information Control System
The recent increase in the number of PIC/S member countries and the tightening of data integrity inspections makes it necessary to ensure the efficient operation and regulatory response of the entire business process related to analytical laboratories. In quality testing of pharmaceuticals and other products, tests are conducted based on predetermined test plans and test conditions, and the results must be appropriately controlled.
LabSolutions™ i-QLinks™ provides integrated control over the quality testing operations of an analytical laboratory, including the preparation of test plans and instructions, the incorporation of test results from analytical instruments such as HPLC, the automatic preparation of test reports from the incorporated test results, and the management of the quality test progress. It also supports data integrity by centrally managing information from test items, analysis sequences, and raw test data. As a result, the reliability of quality testing work can be ensured and the workflow efficiency can be dramatically improved.
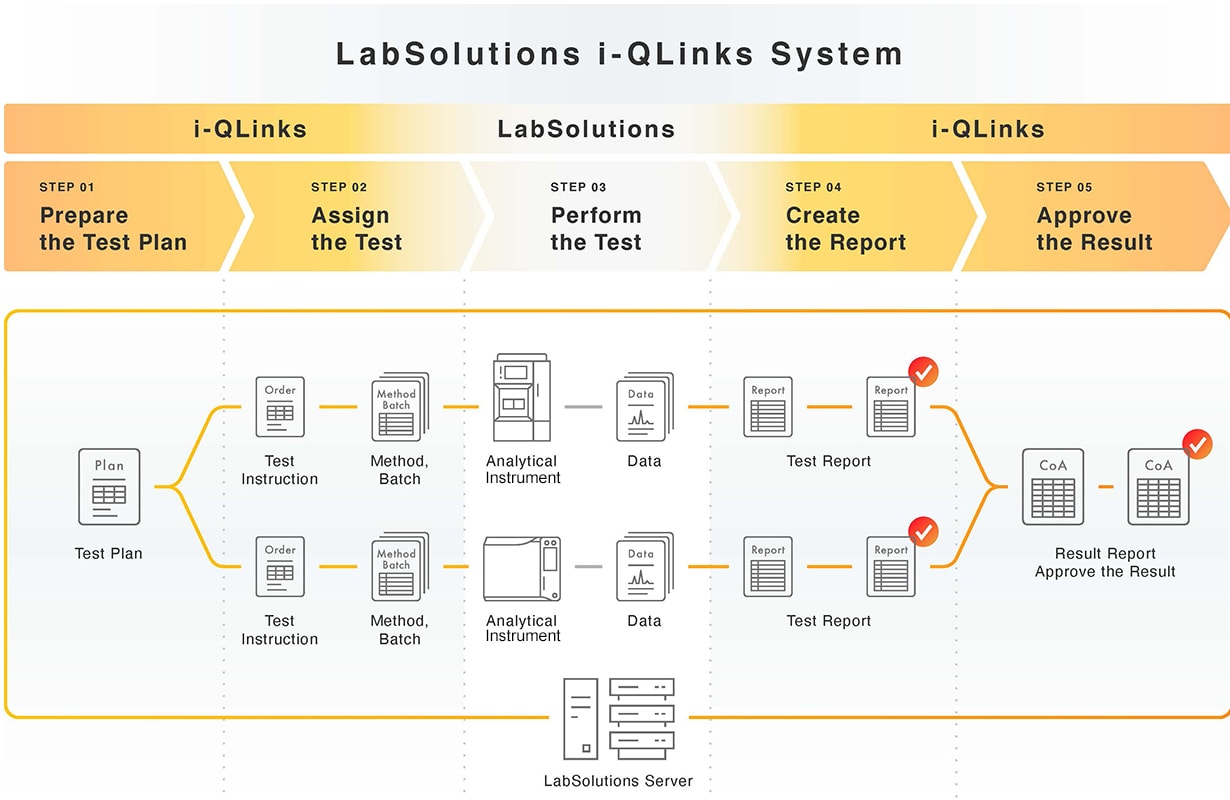
✓ Prepare the Test Plan
The QC manager prepares a test plan based on test request information about the sample.
✓ Assign the Test
The QC manager sets the test to be performed based on the test plan and assigns an operator.
✓ Perform the Test
The operator performs the assigned test.
✓ Create the Report
The operator creates the test report. Based on the test report, the QC manager creates a result report.
✓ Approve the Results
The supervisor confirms and approves the results report.

LabSolutions i-QLinks are a trademark of Shimadzu Corporation or its affiliated companies in Japan and/or other countries.
Windows is a registered trademark or trademark of Microsoft Corporation in the United States and/or other countries.





