This Is The Power Of AI - Discover LabSolutions MD Now
Simultaneous Analysis of Remdesivir and Metabolites in Human plasma Using Fully Automated Sample Preparation LC/MS/MS System
Remdesivir (brand name: Veklury®), which was developed by Gilead Sciences (U.S.) for treatment of Ebola virus disease, is a prodrug having antiviral activity against singlestrand RNA viruses. It is known to be partly metabolized to activated GS-441524, the main metabolite of remdesivir, in vivo1). In Application News C218, we introduced an robust, highly-sensitive simultaneous measurement method using LC/MS/MS with manual pretreatment. Meanwhile, manual pretreatment of plasma samples requires a certain level of workload. This report introduces a method of simultaneously analyzing remdesivir and its metabolite using the automated sample preparation LC/MS/MS system that can reduce variation between procedures, mix-ups of the samples, and risk of exposure to the samples (Fig. 1).
Analysis of Remdesivir in plasma with Fully Automated Pretreatment
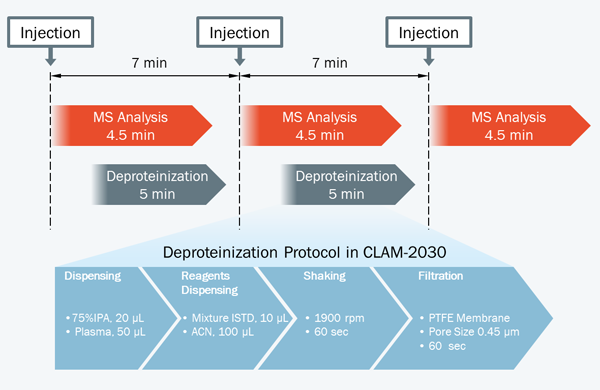
Fig. 2 Workflow of Fully Automated Sample Pretreatment
For analysis of low-molecular compounds in plasma using LCMSTM, it is common to use supernatant collected following deproteinization by adding an organic solvent. With the fully automated sample preparation LC/MS/MS system, these preparatory steps are done automatically just by placing a blood collection tube in the system after plasma separation (Fig. 2). Pretreatment of the next sample can also be performed in parallel with LC/MS/MS analysis, which can greatly reduce the time required to analyze each sample. This analysiswas performed in a per-sample cycle time of seven minutes from plasma pretreatment to the simultaneous analysis of remdesivir and itsmetabolite using LC/MS/MS.
Analysis Conditions and Pretreatment of Samples
![Fig. 3 MS Chromatograms of Remdesivir, [U-Ring-13C6]- Remdesivir (Left) and GS-441524, [13C5]-GS-441524 (Right)](https://www.shimadzu.com/an/sites/shimadzu.com.an/files/d7/ckeditor/an/industry/small_molecule_pharmaceutical/Preclinical/c217/f3.png)
Fig. 3 MS Chromatograms of Remdesivir, [U-Ring-13C6]-
Remdesivir (Left) and GS-441524, [13C5]-GS-441524 (Right)
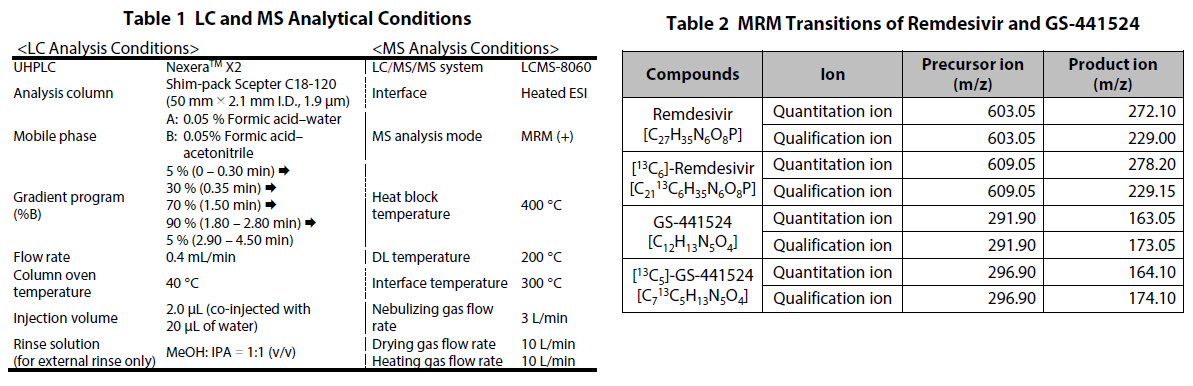
Remdesivir (P/N: C8799*) and GS-441524 (P/N: C8847*), as the target compounds, and [U-Ring-13C6]-remdesivir (P/N: C8845*) and [13C5]-GS-441524 (P/N: C8855*), as their stable isotopes, were purchased from Alsachim, one of the companies of the Shimadzu Group. [U-Ring-13C6]-remdesivir and [13C5]-GS- 441524 were used as materials of the internal standard. To commercially available human plasma treated with EDTA 2K, remdesivir and GS-441524 were added. Following this, the calibration curves and QC samples were prepared. Analysis was performed using the LC and MS analysis conditions shown in Table 1 and the multiple reaction monitoring (MRM) data acquisition parameters shown in Table 2. Shim-pack ScepterTM C18-120 (50 mm×2.1 mm I.D., 1.9 μm) was used as the analytical column. Fig. 3 shows theMS chromatograms. Calibration was performed using 5 calibration points at concentrations of 100, 500, 1000, 2500 and 5000 ng/mL for remdesivir and 5 calibration points at concentrations of 5, 25, 50, 250 and 500 ng/mL for GS-441524 (n = 5 for each calibration point). [U-Ring-13C6]-remdesivir (2.5 μg/mL) and [13C5]-GS-441524 (0.25 μg/mL) were mixed with methanol to be used as the internal standard (ISTD). Samples are automatically prepared through a series of steps. These comprise mixing 20 μL of 75%IPA, 50 μL of plasma, 10 μL of ISTD and 100 μL of acetonitrile, shaking the mixture, and then filtration of the mixture using a PTFE membrane filter, as shown in Fig. 2. Finally, the prepared sample is used for LC/MS/MS analysis.
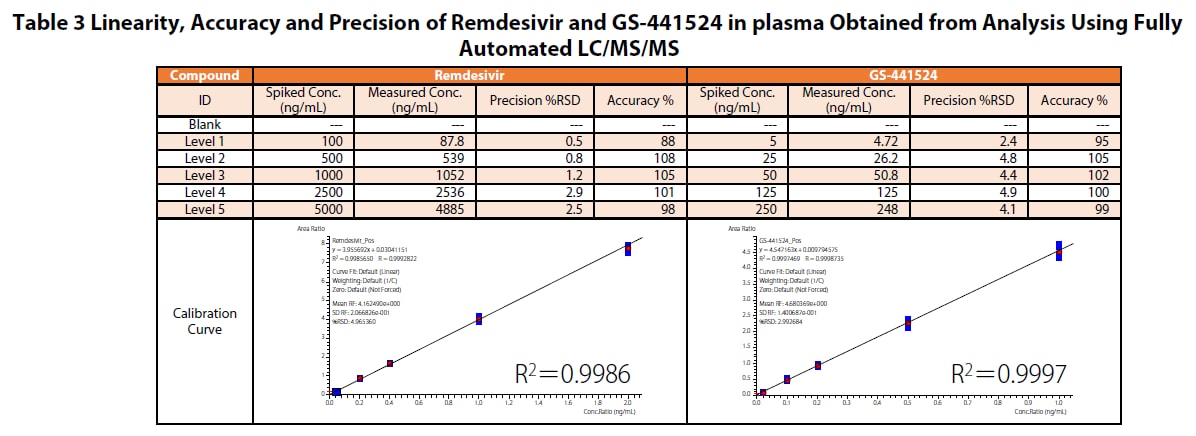
Preparation of Calibration Curves
Calibration curves prepared using the fully automated sample preparation LC/MS/MS are shown in Table 3. Good linearity was obtained in the set calibration range. The precision (reproducibility) of remdesivir and GS-441524 in the entire concentration range, including the quantitative lower limit, was %RSD 0.5 %-2.9 % and %RSD 2.4 %-4.9 %, respectively. Similarly, the accuracy of remdesivir and GS- 441524 was 87.8 %-108 % and 94.5 %-105 %, respectively, indicating that the accuracy of both was within 100 ± 15%.