This Is The Power Of AI - Discover LabSolutions MD Now
Analysis and Evaluation of Chiral Drugs in Biological Samples Using the Nexera UC-MS/MS System
The optimization for chiral separation using supercritical fluid chromatography (SFC) starts from employing column scouting to find the column and mobile phase appropriate to separation.
This article introduces an example of the selectivity and sensitivity of drug level monitoring in a biological sample and the evaluation results of the analysis method, as an application to the pharmacokinetics research of chiral separation using SFC/MS/MS, after having selected an appropriate column.
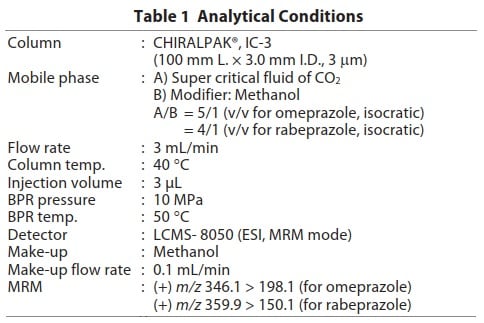
The applicability of human plasma matrix to SFC was evaluated taking an example of enantiomeric drug omeprazole, well-known as a proton pump inhibitor. Fig. 1 shows the pretreatment procedure employed for the blood plasma sample. Table 1 lists the analytical conditions. CHIRALPAK® IC-3 from Daicel Company, which exhibited good separation when utilized in Application News No. L495 was used as the column. Detection was performed using the LCMS-8050 triple quadrupole mass spectrometer
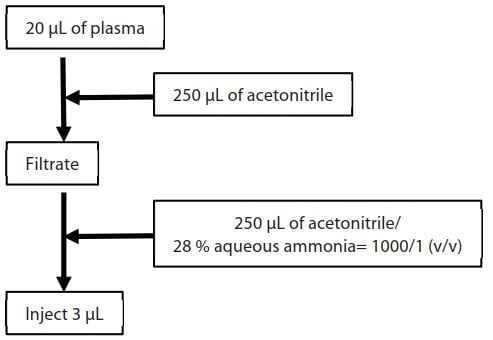
Fig.1 Plasma Sample Pretreatment Procedure
Nexera UC
Features:
Nexera UC improves your analytical workflow by utilizing a completely new separation technology, Unified Chromatography, which unites sample separation, analysis with various separation modes, and high-sensitivity detection.


