This Is The Power Of AI - Discover LabSolutions MD Now
High-Speed Analysis of Pramipexole following the Draft Guidance of International Harmonization of Pharmacopoeias
In recent years, ultra-high performance liquid chromatography (hereinafter, UHPLC) has been widely adopted in the pharmaceutical field to improve efficiency and productivity in analytical work. Responding to these situations, General Chapter <621> CHROMATOGRAPHY in the 40th Edition of the United States Pharmacopeia (USP) and “Adjustment of chromatographic condition” in the 8th Edition of the European Pharmacopeia (EP) allow changes in high speed analysis conditions within a range that conforms to the system suitability test. On the other hand, the Japanese Pharmacopeia (JP) does not specify a clear allowable range for changes in analytical conditions, and as of this writing (March 2018), both USP and EP essentially do not allow high-speed gradient separation.
In this situation, the Japanese, US and European Pharmacopeias are working toward international harmonization, and there is a movement to unify the allowable ranges of analytical conditions. In this movement, the new allowable range of gradient separation is also expected to be specified. (1)
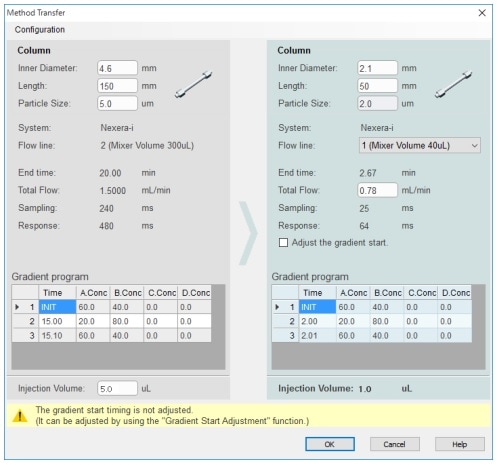
This article introduces an application of a high speed analysis of pramipexole dihydrochloride analogs,(2) as described in the 40th Edition of USP, using Shimadzu integrated LC system Nexera™-i MT based on the draft guidance of international harmonization of JP/USP/EP. It should be noted that this draft is based on the draft version of the international harmonization guidelines for public comment published in July 2017, which may differ from the final version.
Nexera-i MT
Features:
The Nexera-i MT is a new addition to the i-Series Plus lineup. It enables both conventional analysis via its high compatibility with existing LC systems and rapid analysis via high-speed methods. In pharmaceutical development, a single integrated system allows process synthesis screening analysis for synthetic substances in the UHPLC flow line, and impurity content identification in the HPLC flow line.