This Is The Power Of AI - Discover LabSolutions MD Now
LC/MS/MS Analysis of Impurities in Active Pharmaceutical Ingredients Using the Co-Sense for Impurities System
Detection of impurities in active pharmaceutical ingredients (APIs) is often conducted using an HPLC-UV method. However, qualitative and quantitative analysis of impurities requires not only the separation of the impurities from the major component, but also separation among impurities themselves. The time and effort required to establish effective analytical conditions for this type of analysis are significant. Furthermore, the source of the impurity, whether it be the sample itself or some external factor associated with a particular lot, must also be determined. Here we demonstrate analysis of an impurity in an API using the 2-dimensional LC/MS/MS separation feature of the Co-Sense for Impurities System.
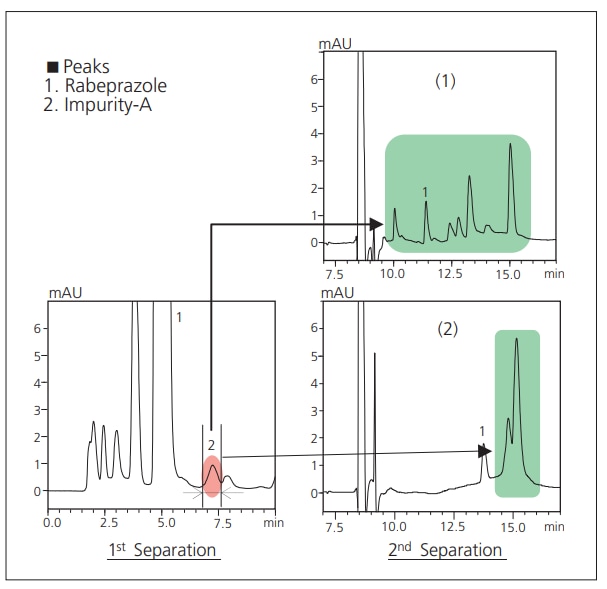
2-Dimensional Separation of Impurity-A Peak


