This Is The Power Of AI - Discover LabSolutions MD Now
Purification

Nucleic acid drugs, such as antisense oligonucleotides, exert their efficacy by interacting with target genes inside and outside cells. Unlike conventional small molecule drugs, they are capable of targeting disease causes at the genetic level and are attracting attention as a next-generation drug. Nucleic acid drugs are mainly produced through chemical synthesis, but the synthesis process also produces many impurities such as shorter length components and protecting groups, so proper separation and purification of the target oligonucleotide is required.
Oligonucleotides analysis by Ion Exchange Chromatography (IEX)
In this article, we introduce an analytical method for the separation of oligonucleotides of different length by ion exchange chromatography, assuming that shorter length components are impurities derived from the synthesis process.
In order to achieve optimal analytical performances an inert HPLC system was used. The Nexera XS inert, which is designed to suppress the adsorption of metal-coordinating compounds containing phosphate groups.
- The sequence of the oligonucleotide to be analyzed is shown in Table1. Target oligonucleotides in 20 mer and 4 sequences that were deleted from n-1 to n-4 on the 3’ terminus of target were prepared as impurities derived from the synthesis. All of them were unmodified single-stranded DNA and synthesized by a solid phase synthesis (HPLC purification). These 5 sequences were diluted to 5 μmol/L with water, and an oligonucleotide mixture was prepared.
-
Table 1 Oligonucleotides
Sequence (5' ---> 3') Length (mer) 1 TCTTGGTTACATGAAA 16 2 TCTTGGTTACATGAAAT
17 3 TCTTGGTTACATGAAATC 18 4 TCTTGGTTACATGAAATCC 19 5 TCTTGGTTACATGAAATCCC 20
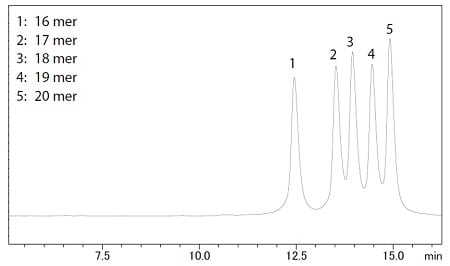
Figure 1 Chromatogram of oligonucleotides mixture
In ion-exchange chromatography, these parathion is based on the number of phosphate groups in the oligonucleotide, i.e., the difference in negative charge. Therefore, generally, shorter oligonucleotides are eluting first. Figure 1 shows a chromatogram of a five-sequence oligonucleotide mixture. Each oligonucleotide was separated by their length.
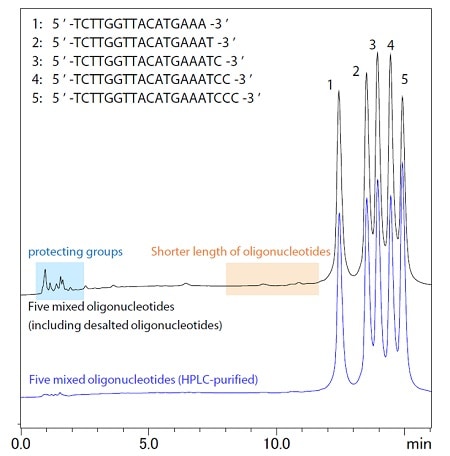
Figure 2 Chromatograms of the oligonucleotide mixture containing impurities
A mixture of five oligonucleotides, at 5 μmol/L concentration each, was prepared (four of them were HPLC-purified while 1 was only desalted) and compared with the mixture of all HPLC-purified nucleotides (Figure2). The target oligonucleotides were completely separated from impurities such as free protecting groups and shorter length oligonucleotides.
When oligonucleotides are analyzed using ion exchange chromatography, the samples are separated and eluted using a salt concentration gradient. In the case of a mobile phase with high pH (basic), the absorption of carbon dioxide from the air can change the pH level. For ion-exchange chromatography even as light change in the pH can have a significant impact on the analytical results (the separation mechanism is based on the charge difference of the analytes). For this reason, it is important to prevent any changes in the pH of mobile phases in order to obtain stable analytical performances. In this article, the effect of changes in mobile phase pH on analytical results is also reported.



