Evaluation of Photoreaction Quantum Yield of Supermolecular Complex (QYM)
The photoreaction quantum yield is one performance indicator for photochemical reactions or photocatalysts. It is the ratio between the number of reaction product molecules from a photochemical reaction (or alternatively the reduction in number of molecules in the substrate) and the number of photons absorbed.
Conventionally, chemical actinometers based on substances with known quantum yield, such as iron oxalate, were used. However, this method requires performing experiments for long periods in a dark room by skilled personnel and also requires repeating the experiments if irradiation parameters are changed. It also has other problems, such as not being able to compensate for changes in light absorption by samples due to reactions.
Therefore, we developed the QYM-01 photoreaction quantum yield evaluation system which permits accurate and easy quantitation measurements of absorbed photons in conjunction with Professor Osamu Ishitani, Graduate School of Science and Engineering, Tokyo Institute of Technology. In this example, we confirmed how results from the new system correlate with the conventional method.
This instrument is not available in Europe and may not be available in some other countries. Please contact your local Shimadzu representative for availability.
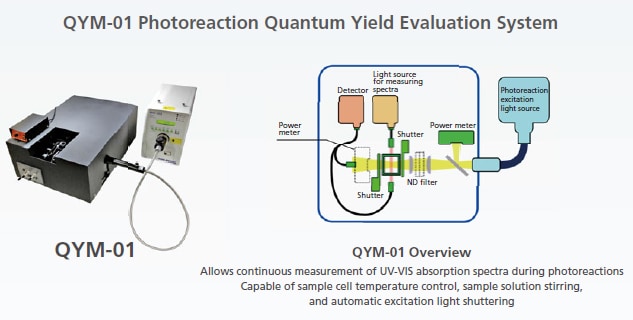
Accurately and Easily measurement of the number of photons absorbed by Sample Solutions
Allows simultaneously measuring changes in UV-VIS absorption spectra in photoreaction solutions
Features
Accommodates a wide range of photoreaction excitation light conditions (wavelength and light level).
- Allows changing the excitation wavelength and measuring the number of absorbed photons anywhere between 250 nm to 800 nm.
- The excitation light level can be adjusted and set by adjusting the number of photons irradiated.
Easy Measurements
- Includes a built-in spectrometer which has been calibrated using a NIST (National Institute of Standards and Technology)-traceable actinometer of which absolute light quantity is managed.
- Eliminates the need for calibration using a chemical actinometer.
- The excitation light level and wavelength switching is controlled via computer software.
- Includes simple computer software. Measures the number of photons using optimal measurement parameters.
Supports accurate measurements.
- Simultaneous UV-VIS absorption spectra measurement capability, a correction function for changes in the light level of the excitation light source, and other features ensure the number of absorbed photons can be measured accurately.
- Displays the photo count in real time. Allows confirming the current measurement status.
Analytical Data
Measurement of Quantum Yield of CO2 Reduction Reaction by Ru-Re Supermolecular Complex Photocatalyst
We measured the quantum yield of a carbon dioxide reduction reaction by a Ru-Re supermolecular complex photocatalyst. Absorbed photons were measured using the QYM-01 and the amount of carbon monoxide generated from the reduction reaction was quantitated using a gas chromatograph. The quantum yield reported for carbon monoxide assuming the experimental conditions used was 0.151). The experiment resulted in a quantum yield of 0.16.
| Photocatalyst | Ru-Re (FPh) (From Tokyo Chemical Industry, Product No. R0100, used without purification) | |
| ReactionConditions | PhotocatalystDonor reducing agentSolventSolution volumeIrradiation lightReaction vessel | Ru-Re (FPh) (0.3 mM)BNAH (0.1 M)DMF-triethanolamine (5:1 v/v solvent mixture)4 mL480 nm xenon lampQuartz cell with branch (4 polished windows)(11 mL volume, 7 mL gas phase, 4 mL liquid phase) |
| OperatingProcedure | Transfer the prepared solution to the 4-sided quartz cell with branch using a 4-mL transfer pipette.Bubble with CO2 for 30 minutes and then seal the cell with a septum (prepare 3).Then after irradiating with light for 1, 2, or 2.5 hours, use a gas-tight syringe to acquire 100 μL of gas from the gas phase and quantitate the CO by GC. | |
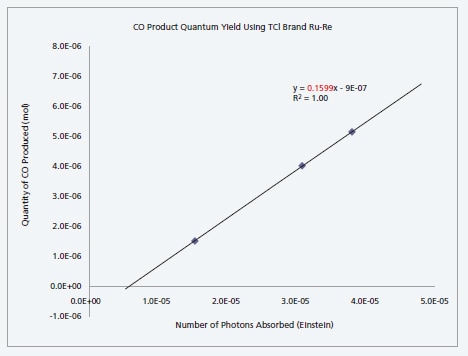
| Quantum Yield Measurement Result | 0.16 |
| Reference Document | 0.15 |
|
References 1) |
Y. Tamaki, K. Watanabe, K. Koike, H. Inoue, T. Morimoto, O. Ishitani, Faraday Discuss. 2012, 155, 115. |
|
Source: |
Ishitani–Maeda Laboratory, Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology. |
Comparison of QYM-01 and Chemical Actinometer (Iron Oxalate) by Measuring Light Intensities
The QYM-01 was verified by comparing measurements of the absorbed light intensity by the QYM-01 and a chemical actinometer. The QYM-01 was used to irradiate an aqueous potassium trioxalatoferrate (III) sample with photoreaction excitation light and measure the photons absorbed. Then the number of photons absorbed was determined from the amount of iron (II) produced in solutions with different irradiation periods in accordance to the relevant section in the fifth edition of "Jikken Kagaku Koza" (Series of Experimental Chemistry), published by the Chemical Society of Japan.
| Experiment 1 | Experiment 2 | |
| Excitation Light Wavelength
Sample ConcentrationPhoton Yield(value indicated in the fifthedition of "Jikken Kagaku Koza")
|
365 nm (Xenon lamp)6 mM1.22 | 480 nm (Xenon lamp)150 mM0.94 |
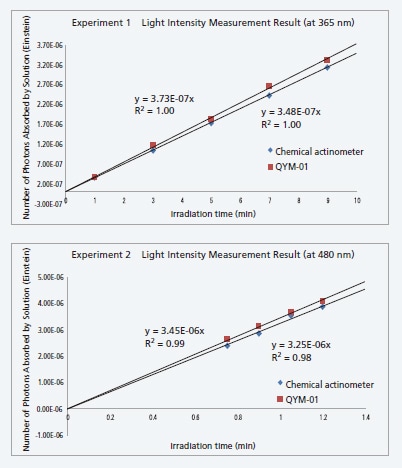
| Number of Absorbed Photons (Einstein/s) | ||
| Experiment 1 | Experiment 2 | |
| Chemical Actinometer QYM-01 |
5.84 × 10−9
6.19 × 10−9
|
5.40 × 10−8
5.78 × 10−8
|
Source: Ishitani−Maeda Laboratory, Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology.


