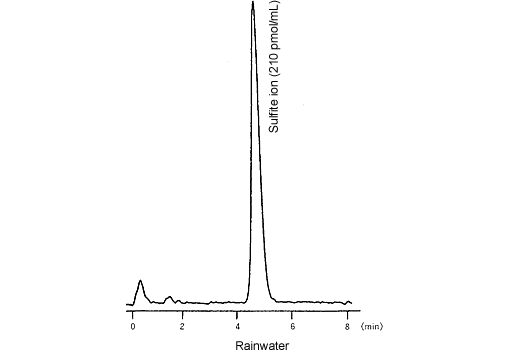
Sulfite Ions in Rainwater
The sulfurous acid contained in factory emissions and automobile exhaust gases is one of the substances causing acid rain. The Rankine method and ion chromatography are well known for the quantitative analysis of sulfurous acid. However, both these methods suffer from problems with operation, sensitivity, and measurement accuracy.
The newly developed derivatization-fluorescence detection method is introduced below. With this method, to suppress decomposition during sample treatment and separation, the sulfurous acid is reacted with formaldehyde to create stable hydroxymethane sulfonic acid. After separation, this is reacted in the presence of orthophthalaldehyde (OPA) and primary amines to form an isoindole derivative that can be detected by fluorescence detection, which allows the highly accurate and sensitive quantitation of unstable sulfite compounds.
Reference
M. Takahashi, M. Nishimura, M. Hayashi, Shimadzu Review, Vol. 54 No. 4 (1997)
High-Performance Liquid Chromatograph

This separation/analysis instrument is widely used for the quantitative analysis of inorganic ions. Ion chromatographs are available as a suppressor type that allows ultra-high-sensitivity analysis to the ppb level and as a non-suppressor type that supports diverse applications.


