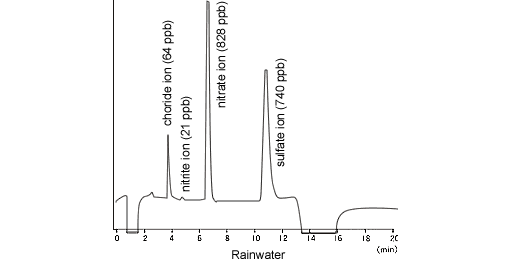
Anions in Rain Water (Acid Rain)
The acidification of rain has become more severe in recent years and its effects on the ecosystem are causing problems. The main causes of acid rain are SOx and NOx in the atmosphere, which convert to sulfuric acid (H2SO4) or nitric acid (HNO3), respectively, in rainwater. The ion chromatograph is suited to the measurement of these ions, as it offers highly sensitive simultaneous analysis of multiple ions.
Ion Chromatograph

This separation/analysis instrument is widely used for the quantitative analysis of inorganic ions. Ion chromatographs are available as a suppressor type that allows ultra-high-sensitivity analysis to the ppb level and as a non-suppressor type that supports diverse applications.


