What is a DSC?
Differential Scanning Calorimeter
A Differential Scanning Calorimeter (DSC) measures temperatures of a reference material and a sample while changing the sample temperature in accordance with a program, and then measures the amount of heat from the temperature difference.
Melting, Glass Transitions, Crystallization, Curing Reaction, Examination of Thermal History, Specific Heat
Principle of DSC
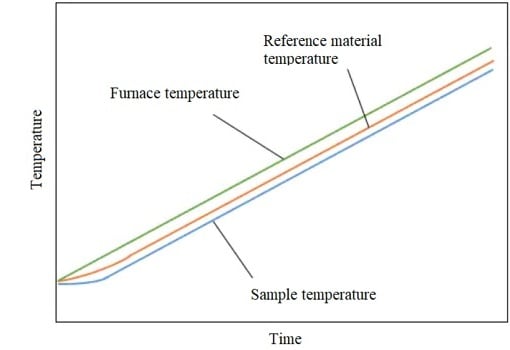
When a furnace is heated, a sample and a reference material are heated at a slightly slower rate than the furnace temperature.
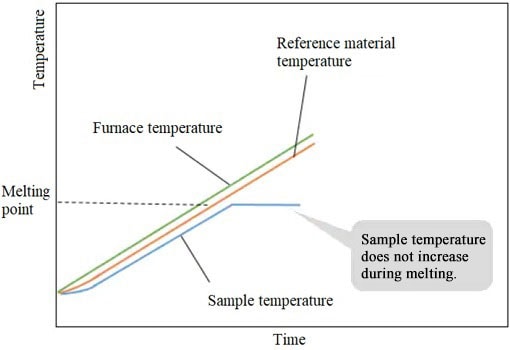
When the sample starts to melt, the sample temperature stops rising (because of the heat used for melting). However, the temperature of the reference material increases.
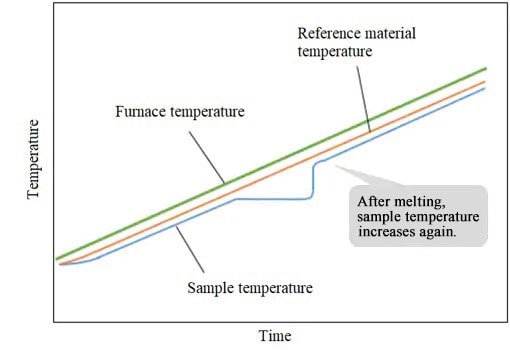
After melting, the sample temperature rises again following the furnace temperature.
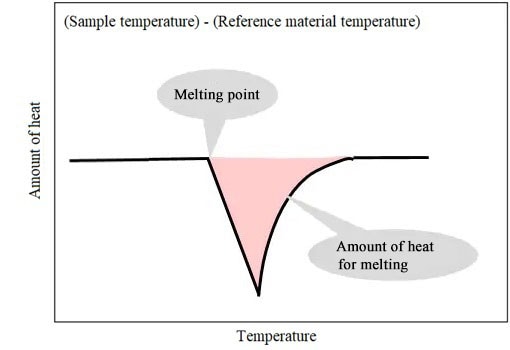
(Sample temperature) - (Reference material temperature) corresponds to DSC signals.
Sample data of DSC
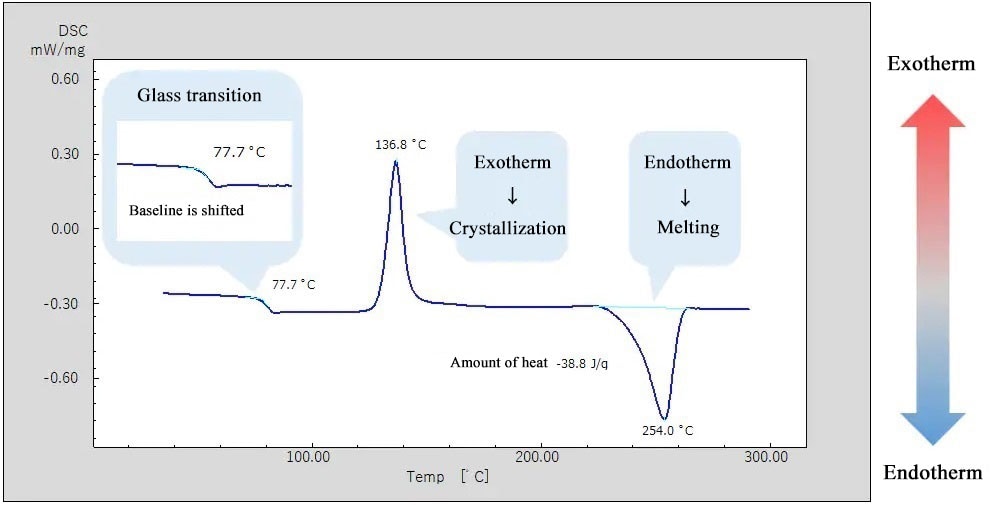
Thermal and mechanical properties of macromolecular materials are known to vary depending on the thermal history of the various samples. The above shows the measurement result for PET, a macromolecule, after it was heated and quenched.
The DSC curve shows a shift of the baseline around 77°C, indicating "glass transition". Also, an exothermic peak is observed around 130°C, indicating an exothermic reaction caused by crystallization. The endothermic peak observed at around 250°C refers to an endothermic reaction by "melting". Crystallization after glass transition and subsequent melting were observed, indicating that the sample was in an amorphous state with little crystallization by quenching after heating.
For this reason, the thermal history of materials can be confirmed by measuring with a DSC (differential scanning calorimeter).






