What is Supercritical Fluids Chromatography?
Chromatography in General
Chromatography is the collective term for various techniques used to separate target components in mixture samples, either chemically or physically, so that they can be qualitatively or quantitatively analyzed, collected, or for other purposes. In chromatography techniques, components are separated within two phases, a mobile phase and a stationary phase. If a gas is used as the mobile phase, the technique is referred to as gas chromatography (GC). If a liquid is used, it is referred to as liquid chromatography (LC). Both GC and LC have become essential techniques for analyzing organic compounds.
LC systems that include a pump for pumping the mobile phase, an autosampler for automatically loading samples, and other devices for increasing performance are referred to as high-performance liquid chromatograph (HPLC) systems. In recent years, there has been increasingly widespread use of ultra high-performance liquid chromatograph (UHPLC) systems. These systems can sustain very high back pressures generated by use of sub 3u particles as stationary phase. However, low particle size results in ultra fast analysis with and sharper peak shapes.
Supercritical Fluid Chromatography
Research on supercritical fluid chromatography (SFC) as an analytical technique began in the 1960s. Typically, SFC refers to chromatographic technique involving using supercritical carbon dioxide as the mobile phase. Since SFC uses CO2 like a liquid, it is also referred to as “LC using CO2.”
Before commercialization of SFC, measuring compounds with a wide range of properties required using multiple analytical techniques, however using a combination of SFC and LC for “unified chromatography” has now enabled simultaneous analysis of heterogeneous components repeating.*1
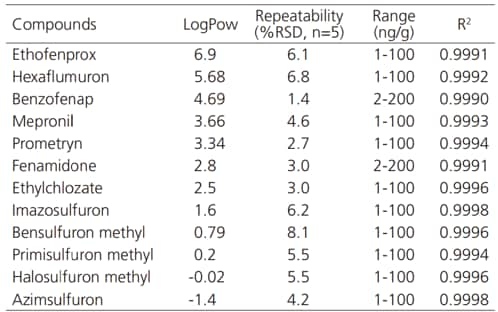
Fig. 1 shows a simultaneous analysis of 510 standard pesticides in single injection using SFC. Table 1 shows the octanol-water partition coefficient, repeatability, and linearity results for few target pesticides. The technique enables accurate simultaneous analysis of compounds with a wide range of polarities.
-
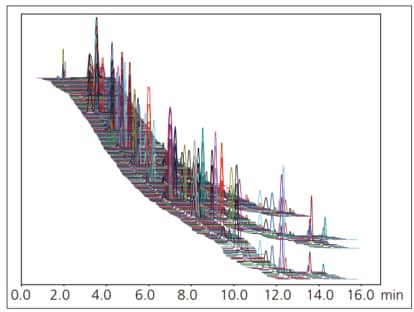
Fig. 1 Mass Chromatogram of Standard Pesticide Mixture Solution
-
Table 1 Repeatability and Linearity for Pesticide Analysis
Learn more
Instrumentation for Supercritical Fluid Chromatography
SFC systems involve roughly the same configuration of instruments as HPLC systems. Fig. 2 shows a comparison of flow diagrams for SFC and HPLC systems. The four major differences between SFC and HPLC configurations are described below.
- Pumps Designed Specifically for Pumping Supercritical Carbon Dioxide
The carbon dioxide used for SFC is liquified by cooling. Therefore, the delivery pump must have built-in cooling functionality.
- Back Pressure Regulator (BPR)
A unit is required to keep the CO2 in a solvent state (liquid or supercritical fluid) within the system and prevent it from vaporizing by maintaining the pressure level within flow channels. In an SFC system, the BPR unit is positioned downstream from a UV, PDA, or other detector or upstream from an evaporative light scattering detector (ELSD) or mass spectrometer (MS).*2 The BPR unit detects pressure in the flow channels and then rapidly opens and closes valves to maintain a constant pressure within the flow channels.
- Modifier Delivery Pump
For SFC analysis, an organic solvent such as methanol or acetonitrile (modifier) is pumped for mixing with the supercritical carbon dioxide. That means separate pumps are required for pumping the supercritical carbon dioxide and modifier.
- Make-up Delivery Pump
If an ELSD or MS unit is used for detection or if the system is used for preparative separation, then a solvent (make-up solvent) is pumped to prevent precipitation in the flow channels or to improve the recovery rate of components in separated fractions.
Make-up solvent is also pumped to improve sensitivity during MS detection, because supercritical carbon dioxide does not promote ionization.
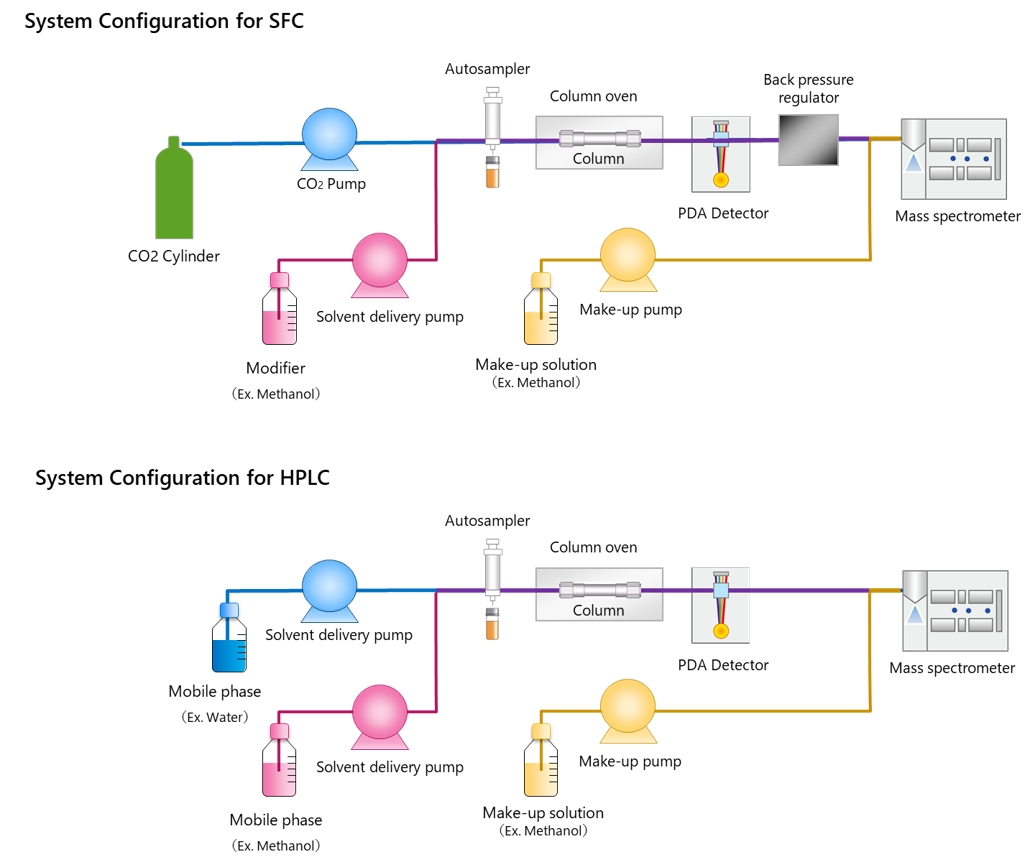
Fig. 2 Comparison of HPLC and SFC System Configurations
- Simultaneous analysis for water- and fat-soluble vitamins by a novel single chromatography technique unifying supercritical fluid chromatography and liquid chromatography J Chromatogr.A 2014 Oct3; 1362:270-7
- Overview of the retention in subcritical fluid chromatography with varied polarity stationary phases J Sep Sci. 2008 May; 31(8):1238-51






