Properties of Supercritical Fluids
Physical Properties of Supercritical Fluids
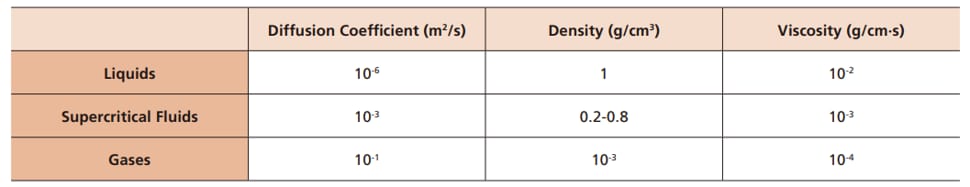
Substances can change their state between solid, liquid, and gas states depending on the temperature and pressure. Vaporization and sublimation are phenomena that occur when heat activates the thermal motion of solid or liquid molecules in a substance, to an extent that the molecules break free from the intermolecular forces keeping the substance in its current state. For example, solid CO2 (dry ice) in a sealed container sublimates to reach a supercritical state under high-temperature high-pressure conditions (Fig. 1). Supercritical state is when substance have properties of both gas and liquid, and they are thus called as Supercritical Fluid. This point in phase diagram with corresponding conditions of temperature and pressure is called Critical Point (Table 1). Supercritical fluids have a higher coefficient of diffusion with lower density and viscosity than liquids. Consequently, higher diffusivity results in better distribution into the mobile phase and better separation, and lower viscosity makes SFC a faster method than HPLC.
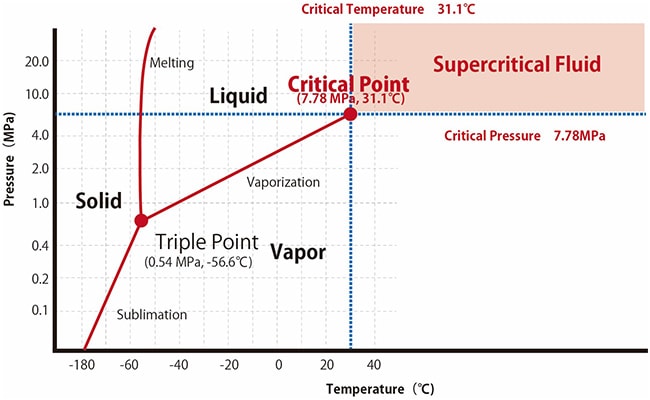
Fig.1 Phase Diagram for Carbon Dioxide
Table 1 Physical Properties of Supercritical Fluids
Supercritical Carbon Dioxide
Critical pressures and critical temperatures for generating a variety of supercritical fluids are shown in Fig. 2. Water is commonly used for high-performance liquid chromatography, but due to its critical pressure of 22.1 MPa and critical temperature of 374 ℃, it cannot be easily kept under conditions necessary for transitioning to a supercritical state. In contrast, supercritical carbon dioxide has a critical pressure of 7.4 MPa and a critical temperature of 31.1 ℃, hence allowing transition to the supercritical state under relatively modest conditions.
Supercritical carbon dioxide offers the following advantages.
- Handling
With critical points at 31 ℃ and 22 MPa, CO2 becomes a supercritical fluid under modest conditions. - Safety
CO2 is not flammable and has minimal risk of explosion even if used compressed. CO2 offers intrinsically low toxicity, when compared to other organic solvents. - Cost
CO2 is mainly sold as a liquid stored in high-pressure cylinders and in most cases can be acquired at a low cost. - Environmental Friendliness
Other organic solvents are incinerated for disposal, which emits large amounts of energy and CO2. However, liquified carbon dioxide gas is collected as a byproduct of fermentation processes or chemical plants. That means using liquified CO2 causes minimal environmental impact.
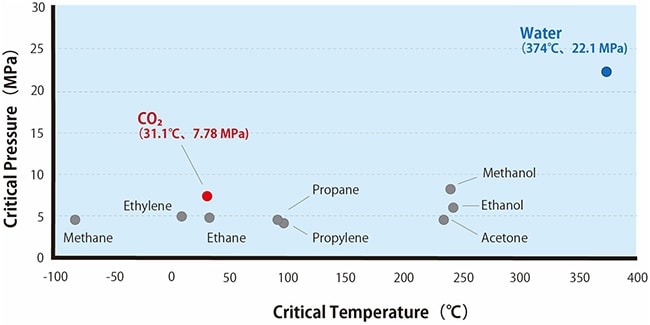
Fig. 2 Conditions for Generating Supercritical Fluids






