Ionization Modes: EI
Features of EI
- EI is the most widely used ionization mode in GC/MS analysis. Almost all commercial GCMS instruments equip this mode as standard ionization.
- EI causes much fragmentation of a molecule whose spectral pattern is useful for identifying sample compound.
- Library search is available. EI mass spectra obtained by 70eV electron bombardment can be used for identification by comparing with spectra registered in the mass spectral library.
- An open type of ion source is used. Vacuum pressure inside the source, mainly determined by carrier gas, is about less than 10-2 Pa.
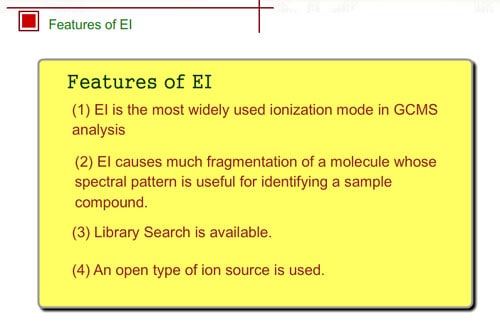
Molecular Ion Produced by EI
In electron ionization, electrons from a filament sometimes knock out an electron of molecules and ionize the same compounds without break-up. The produced ion has the same mass as the original compound except for a negligibly small mass of an electron. We call such an ion a "molecular ion". This ion is very important for determining the molecular weight.
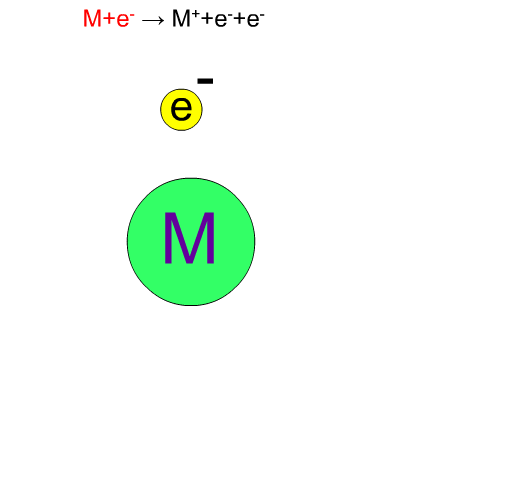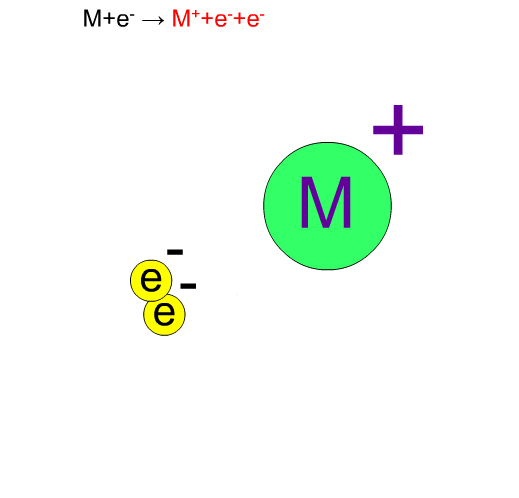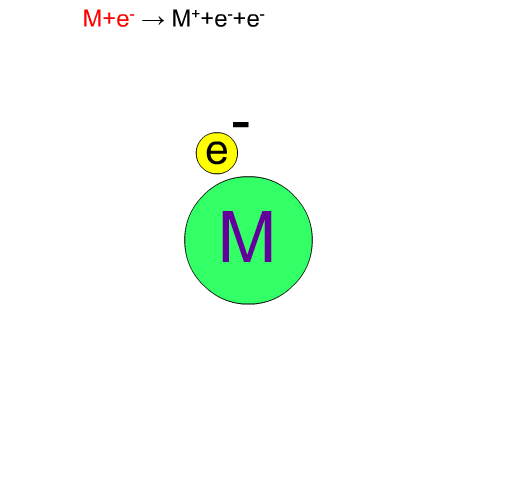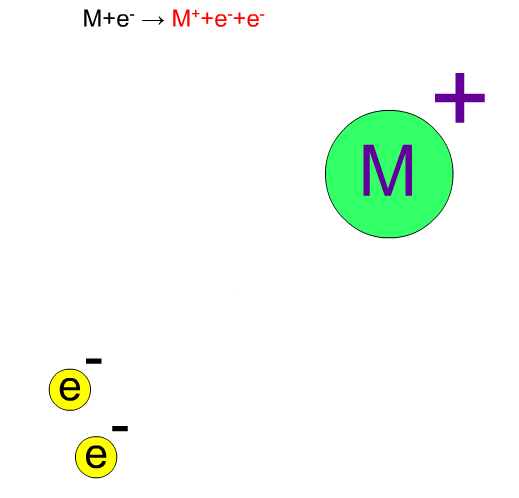
Fragment Ion Produced by EI
70eV-electrons, normally used in EI, have enough energy to break up compounds. We call this decomposition of a molecule "fragmentation". Generally speaking, the molecular ion M+* in a vibration state is first produced, then the fragment ion m+ is produced from this unstable molecular ion because of excess energy in M+*.
Chemical bonds usually break at weak bonds. Analysis of fragment spectra provides us with information on the chemical structures of a compound.

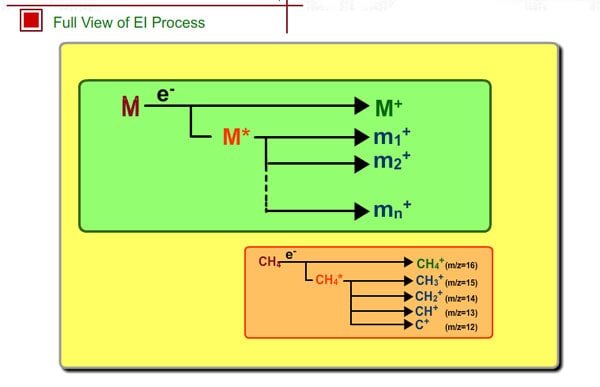
Full View of EI Process
It is said that either a stable molecular ion M+ or an unstable molecular ion M+* is produced by electron impact, the result depending on compounds. For example, straight-chain hydrocarbons with many carbons rarely show molecular peaks, especially for the compounds having many carbons, because the unstable molecular ion M+* easily decomposes into a fragment ion.
For methane, the molecular ion is CH4+. If hydrogen is removed, the fragment ion CH3+ is formed. Formation probability of each ion is constant, so we can get a reproducible spectrum as long as the analytical conditions are the same.
Producing EI Mass Spectrum
This is a simulation for producing a mass spectrum. Ion production is a probability event. Nobody can predict on a head which ion will be created. As the number of ions increase, the spectrum approaches the library spectrum. The truly measured spectrum is obtained by accumulating ions for a given length of time. The longer the time, the better the spectrum.
Electron Energy and Mass Spectrum
The figure shows a spectrum of ethylacetate (MW=88) for 70-eV electrons. Usually for GC/MS analysis, a 70eV electron is used for ionization. A reason to use this energy is that the mass spectra in many libraries, which are frequently used for identification of unknown sample peaks, are customarily measured with 70-eV electrons. When the electron energy is changed, the spectrum changes as per the following:
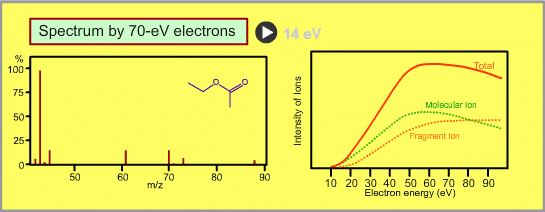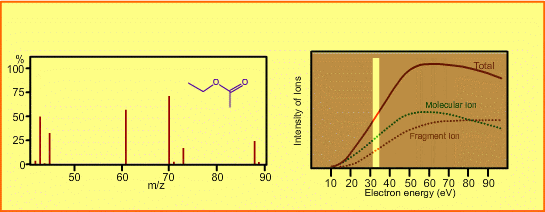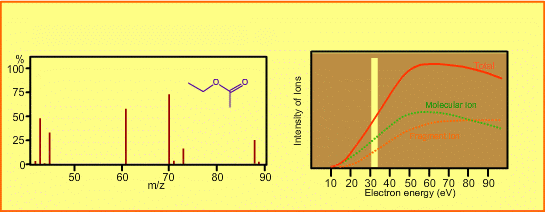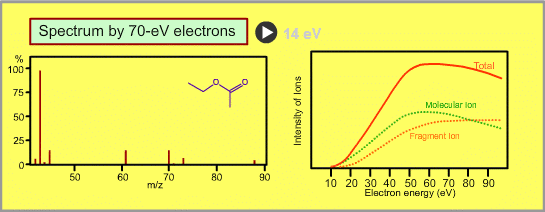
- Ion signal is most intense at around 70eV.
- When the energy is decreased, the spectrum will be less fragmented and the signal intensity seriously decreases. Ions cannot be produced below about 10eV.
LICK"14eV" ("70eV")






