Ionization Modes:NCI
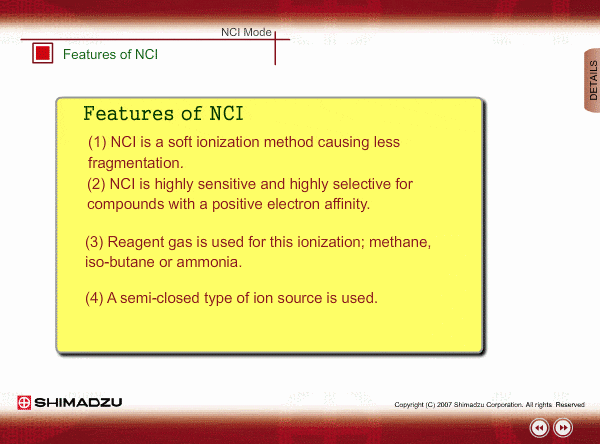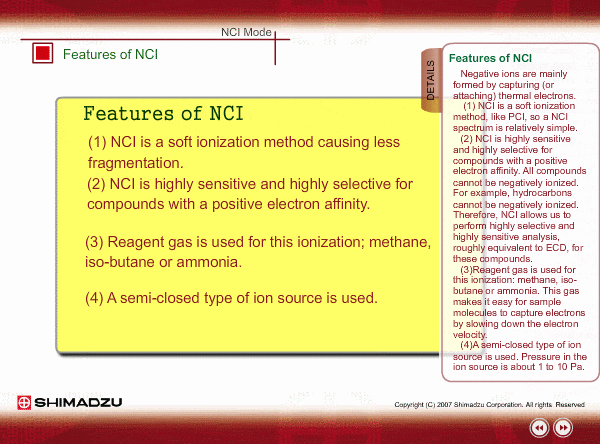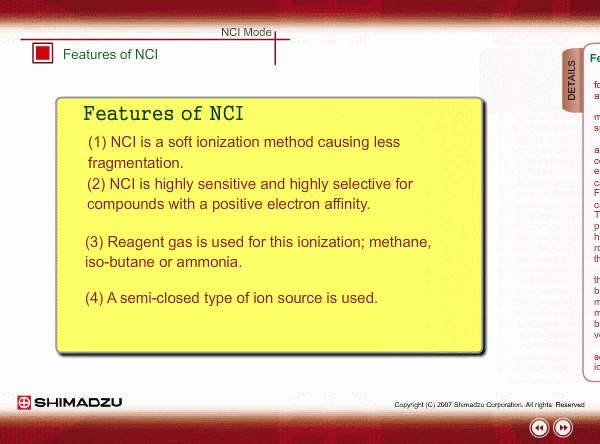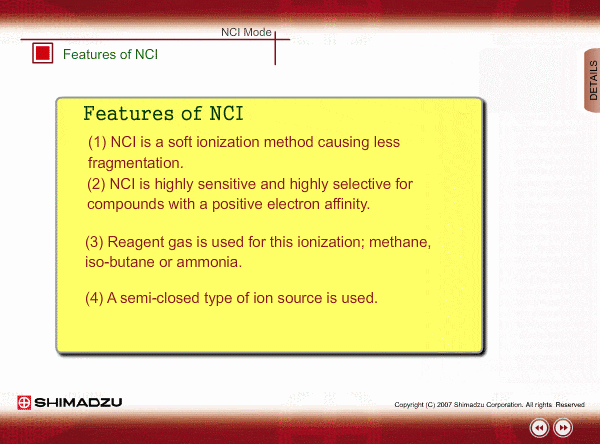
Features of NCI
Negative ions are mainly formed by capturing (or attaching) thermal electrons.
- NCI is a soft ionization method, like PCI, so a NCI spectrum is relatively simple.
- NCI is highly sensitive and highly selective for compounds with a positive electron affinity. All compounds cannot be negatively ionized. For example, hydrocarbons cannot be negatively ionized. Therefore, NCI allows us to perform highly selective and highly sensitive analysis, roughly equivalent to ECD, for these compounds.
- Reagent gas is used for this ionization: methane, iso-butane or ammonia. This gas makes it easy for sample molecules to capture electrons by slowing down the electron velocity.
- A semi-closed type of ion source is used. Pressure in the ion source is about 1 to 10 Pa.
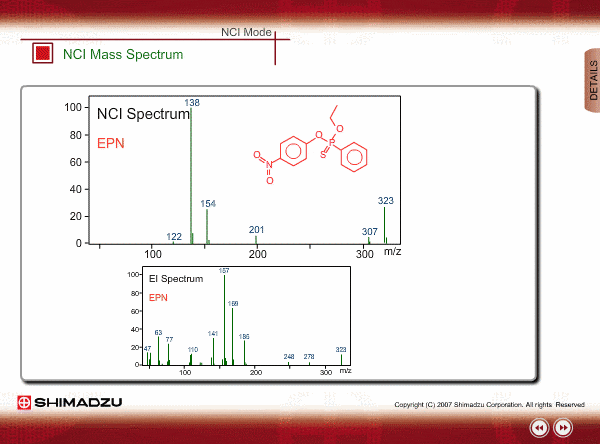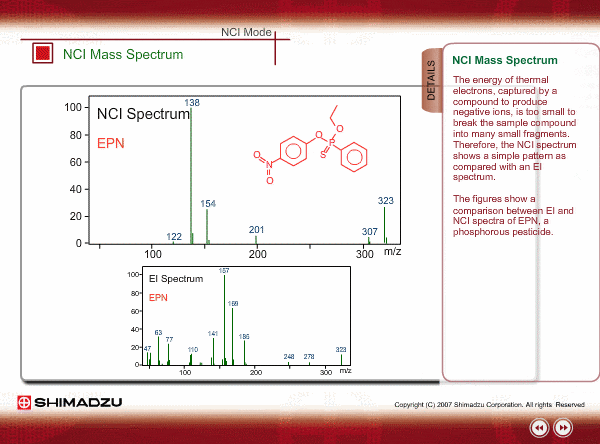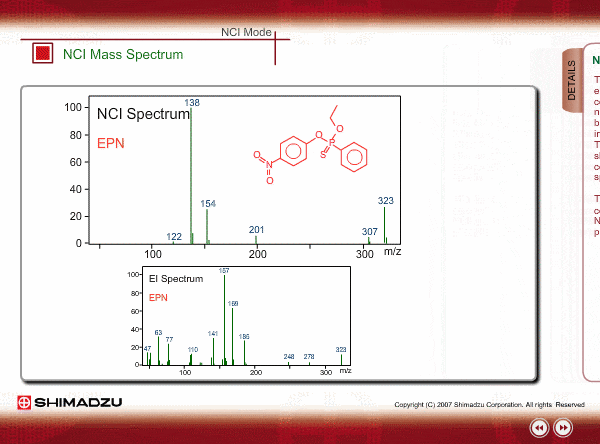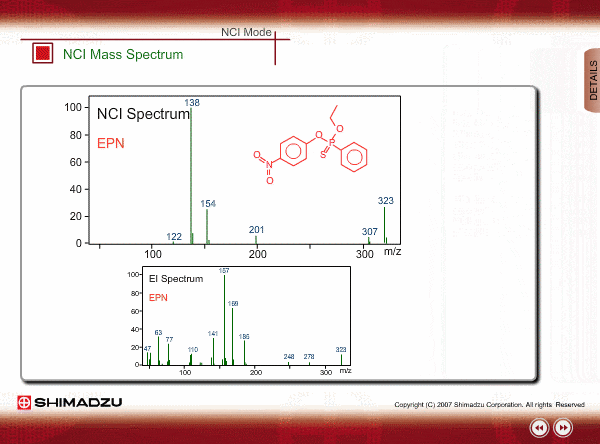
Spectrum of NCI
The energy of thermal electrons, captured by a compound to produce negative ions, is too small to break the sample compound into many small fragments. Therefore, the NCI spectrum shows a simple pattern as compared with an EI spectrum.
The figures show a comparison between EI and NCI spectra of EPN, a phosphorous pesticide.
Ionization Process of NCI
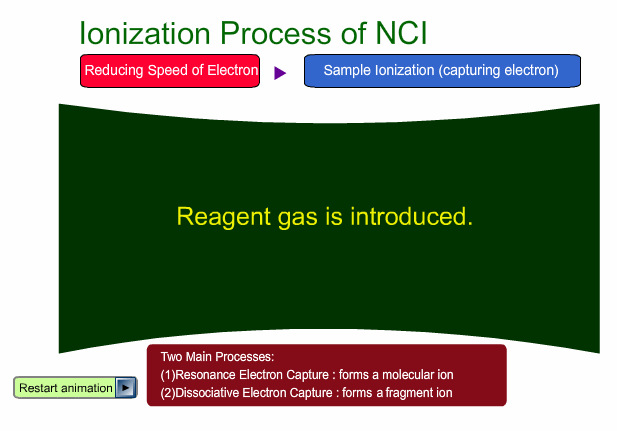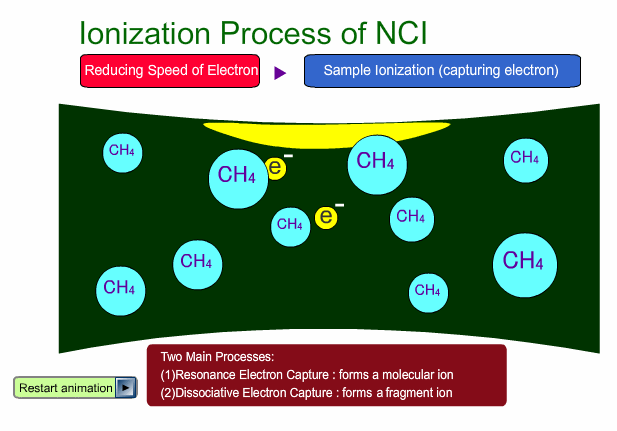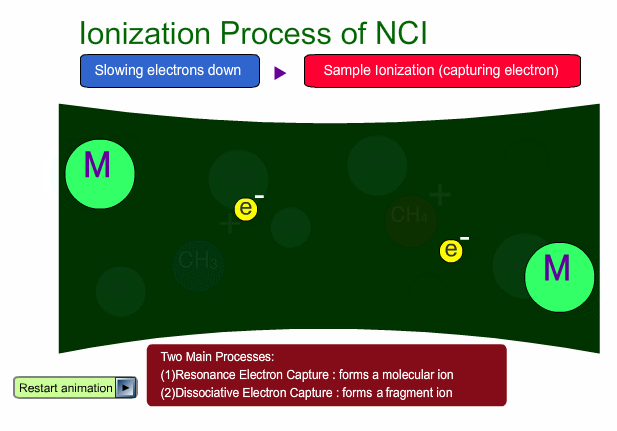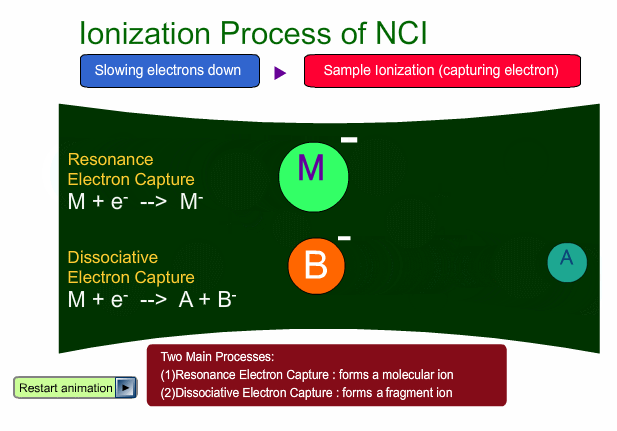
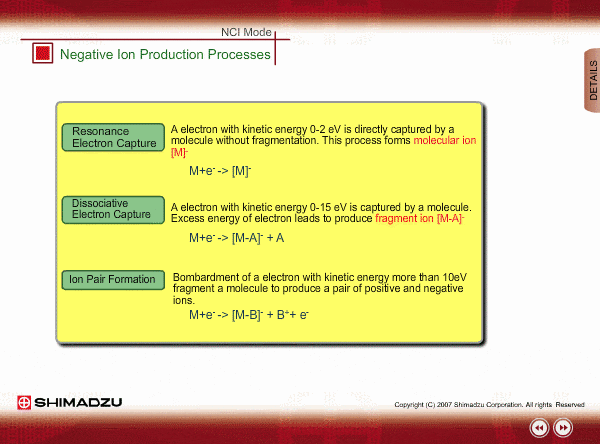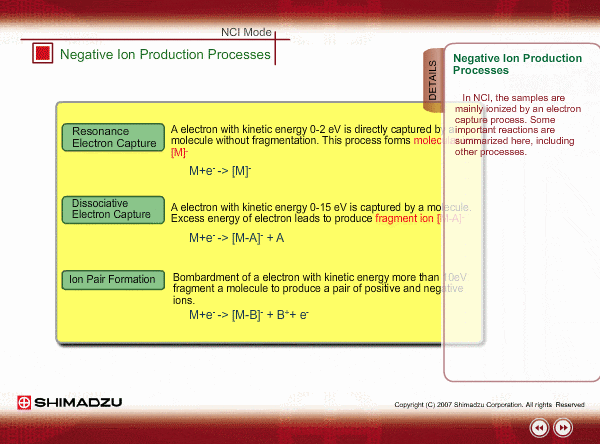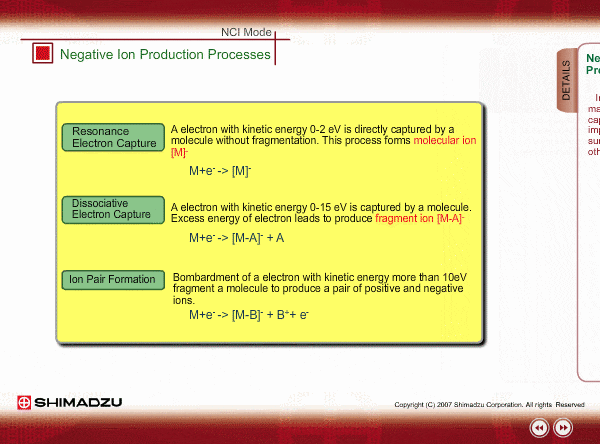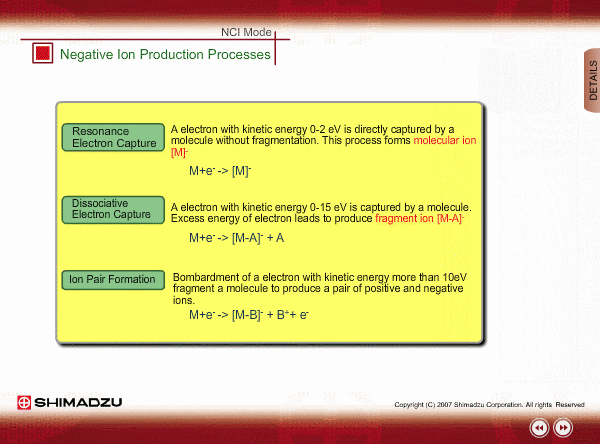
There are two main ionization processes for NCI: resonance electron capture producing a molecular ion and dissociative electron capture causing fragmentation. Electrons emitted from a filament lose their energy to become thermal electrons by collision with reagent gas and ionization of reagent gas molecules. Nearly 0eV electrons are captured by molecules so that molecular ions are produced. If electrons have enough energy to break up molecules, fragmentation occurs.
Molecular Peak and Fragmentation
An NCI spectrum generally shows a simple pattern. The figure is the spectrum of parathion, whose molecular weight is 291. The m/z 169 peak and the 154 peak correspond to the simply fragmented ions.
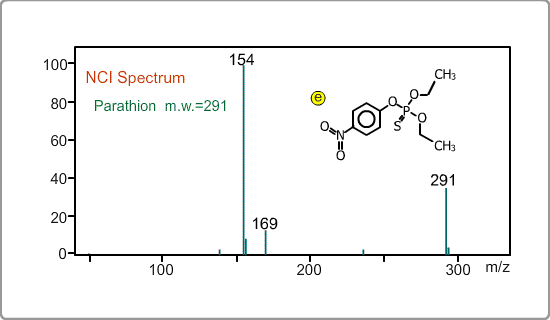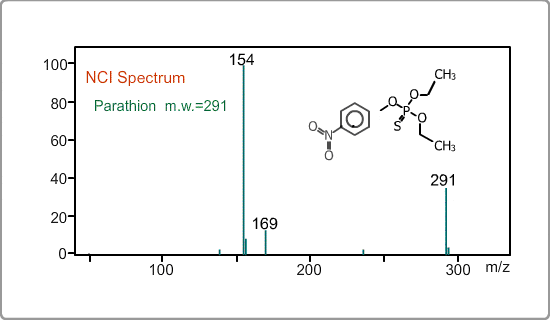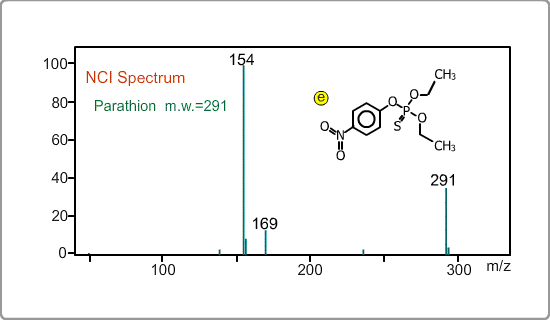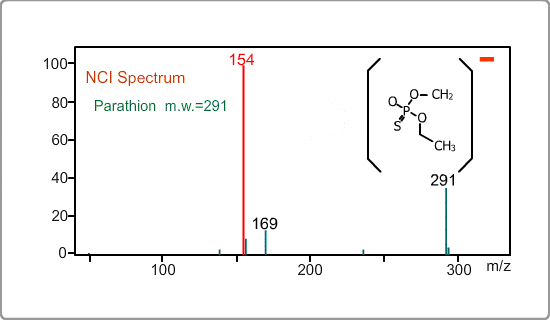
Negative ion Production Processes
In NCI, the samples are mainly ionized by an electron capture process. Some important reactions are summarized here, including other processes.
Useful Application Fields
NCI is suitable for analysis of chlorinated pesticides, organophosphorous pesticides, toxic chlorinated compounds such as PCBs, steroids and drugs, which tend to be captured electrons.







