Scaling Up
(1) Checking the Eluent Composition
When an analysis scale with established conditions that offers problem-free analysis is scaled up for fractioning, problems may occur. Therefore, it is necessary to recheck the eluent.
Depending on the target fraction volume, in some cases it may be necessary to inject large quantities of high-concentration sample to achieve efficient fractioning. Some compounds may dissolve adequately in the eluent at the analysis scale but not at the preparative scale. In such cases, it is important to review the eluent composition and separation mode.
Also, as the fractions are obtained dissolved in the eluent, it is necessary to confirm in advance that no problems will occur during post-processing (concentration, purification, analysis by other instruments, etc.). As evaporative drying may be performed on the fractioned solutions, it is important that the eluent composition contains as few non-volatile salts as possible. Phosphoric acid is often used for analytical HPLC. However, it is often replaced by formic acid, acetic acid, or trifluoroacetate for preparative HPLC. In addition, it is important to avoid using ion-pair reagents in the reverse-phase mode and to use organic solvent-rich rather than water-rich compositions. If a bioassay is to be performed after fractioning, it is also important that the eluent does not contain substances that may inhibit the bioassay (such as highly toxic solvents).
(2) Basic Principles of Scaling Up
Basically, fractioning is performed by simply increasing the size in the analysis conditions, as described below. However, this may lead to problems with inadequate solubility in the eluent with some samples. As described in Determining the Load Limit, confirm that injections of 10 to 100 mg/cm2 are possible by increasing the sample load before scaling up.
When the conventional size is scaled up to semi-preparative or preparative size, if the packing is kept the same, it is thought that the eluent flowrate and sample load can be increased in proportion to the column cross-sectional area to obtain approximately the same separation.
The diagrams below show an example of scaling up using columns with the same packing. A 4.6 mm I.D. column was used at the analysis scale and a 20 mm I.D. column was used at the preparative scale. As the 20 mm I.D. column has approximately 19 times the cross-sectional area of the 4.6 mm I.D. column, the flowrate was scaled up from 0.8 mL/min to 15 mL/min and the sample injection volume was scaled up from 50 µL to 1 mL. This resulted in approximately identical chromatogram patterns.
Therefore, scaling up is easy if a set of columns of different inner diameters but similar packing properties is available.
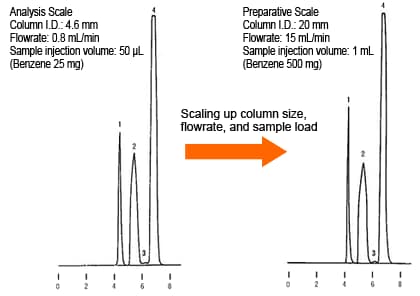
Example of Scaling Up from Analysis Scale to Preparative Scale
1. Benzoic Acid
2. Benzene
4. Naphthalene
Despite the injection volume being increased 20 times from the analysis scale, approximately equal sensitivity and area values are obtained at both the analysis scale and preparative scale (excluding naphthalene). This is thought to occur because the eluent flowrate setting is increased by almost the same ratio, so that the dilution in the eluent results in approximately the same component band concentrations being retained in the detection cell for the same amount of time.
(3) Selecting the Instrument and Column
■ Column and eluent flowrate
Select a column with the appropriate inner diameter by comparing the load limit for the conventional size with the target fraction volume. Determine the eluent flowrate according to the column cross-sectional area by following the Basic Principles of Scaling Up described above.
The table below shows examples of the cross-sectional area ratio and set flowrates scaled up for a variety of columns, based on 4.6 mm I.D. and 1.0 mL/min eluent flowrate.
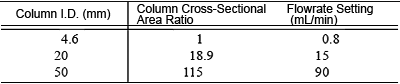
Relationship Between Column Cross-Sectional Area and Flowrate
It may not be possible to use some flowrates shown above with preparative columns in practice, due to issues with the solution viscosity and column pressure resistance. In this case, first determine the flowrate for the preparative column and then convert it back to the flowrate for the conventional column.
■ Solvent delivery units
The table below shows the standard flowrates at analysis and preparative scales and the corresponding solvent delivery units to handle them. Select the solvent delivery pump according to the determined eluent flowrate. A maximum flowrate setting is prescribed for each solvent delivery unit for HPLC. You are recommended to use 1/2 to 2/3 of the maximum flowrate setting as the upper limit for practical applications. (Continuous operation at a high flowrate leads to rapid deterioration of the plunger seal.)

Flow rates and Corresponding Solvent Delivery Units
■ Injectors
The sample injection volume can be scaled up according to the cross-sectional area ratio to select a suitable injector for the injection volume.
If a manual injector is used, a Rheodyne 7725i or 7725 with a range of sample loops of different capacity up to 5 mL, then the manual injector can support both analysis scale and preparative scale. (If the injection volume exceeds 5 mL, the Rheodyne 3725 preparative injector is recommended.)
If an Autosampler is used, the maximum injection volume differs according to the model. The range of injection volume settings also differs according to the options. It may be possible to increase the injection volume of your existing Autosampler by adding an optional sample loop.
| Model Name | Features/Applications | Injection Volume (µL) (maximum, using options) |
Main Wetted Surface Materials |
| SIL-10AF | Permits injection by loop metering method (or syringe metering method). Recommended injection volume range up to 2000 µL. | 1 to 50 (max. 5000) | SS |
| SIL-10AP | Injection volume up to 5 mL is included standard. Suitable for semi-preparative, GPC cleanup, and concentration system applications. | 1 to 5000 | SS |
| SIL-10Ai | Flow lines are made of PEEK, which is resistant to hydrochloric acid and halogens. This model is suited to applications where such an eluent is used. | 1 to 50 (max. 250) | PEEK |
| SIL-20A | Direct-injection system, suitable for conventional size. Excellent reproducibility and accuracy for trace-volume injection. A needle rinsing function is included as standard to minimize cross-contamination (carryover). | 0.1 to 100 (max. 2000) | PEEK, (SS) |
* Details on Autosampler


