Molecular Weight
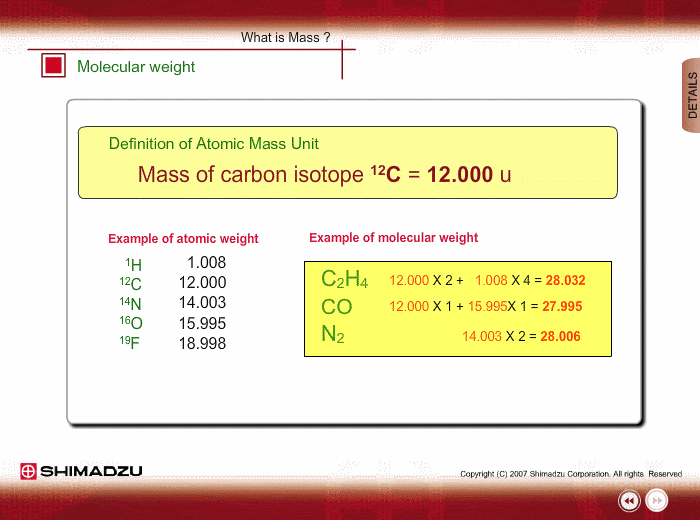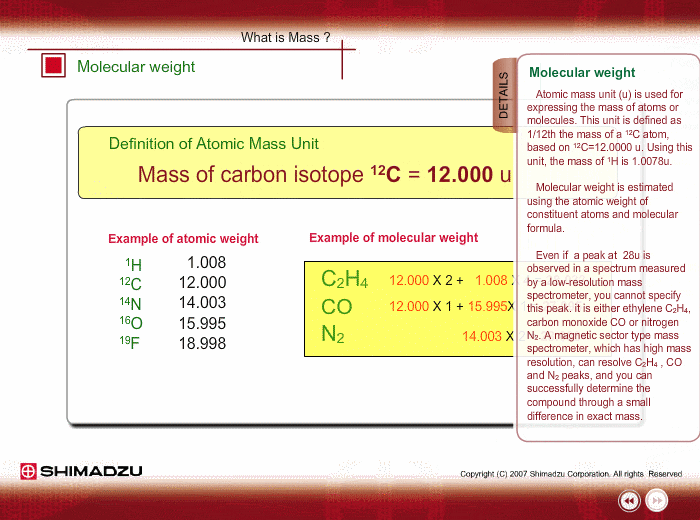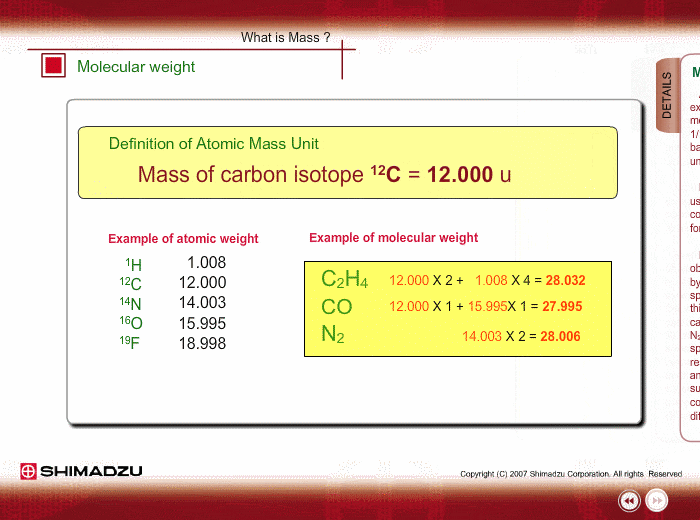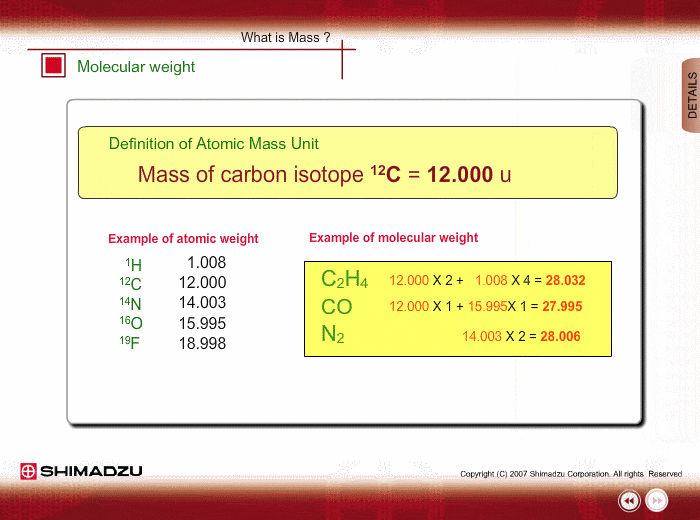
Atomic mass unit (u) is used for expressing the mass of atoms or molecules. This unit is defined as 1/12th the mass of a 12C atom, based on 12C=12.0000 u. Using this unit, the mass of 1H is 1.0078u.
Molecular weight is estimated using the atomic weight of constituent atoms and molecular formula.
Even if the peak at 28u is observed in a spectrum measured by a low-resolution type of mass spectrometer, you cannot specify this peak. It is either Ethylene C2H4, carbon monoxide CO or nitrogen N2. A magnetic sector-type mass spectrometer, which has high resolution in mass, can resolve C2H4, CO and N2 peaks, and you can successfully determine the compound through a small difference in exact mass.
This is the unit in which atomic masses are measured, defined as 1/12 the mass of Carbon 12. The mass of one Carbon 12 atom is exactly 12 u. The atomic mass of hydrogen is 1.007825.
TOP << What Is Mass ?


