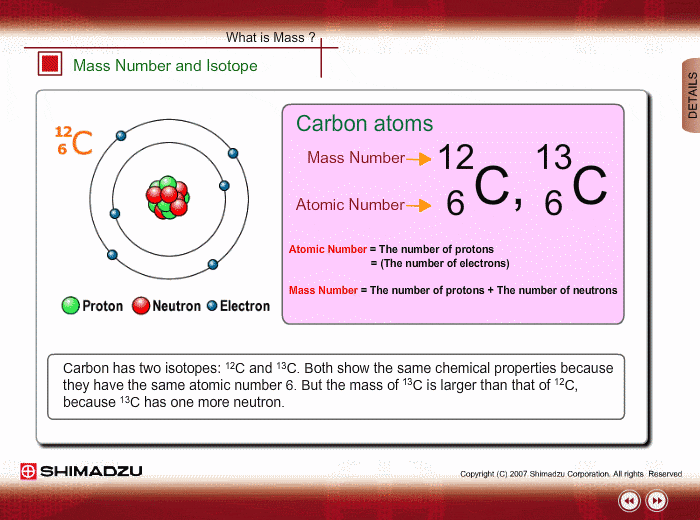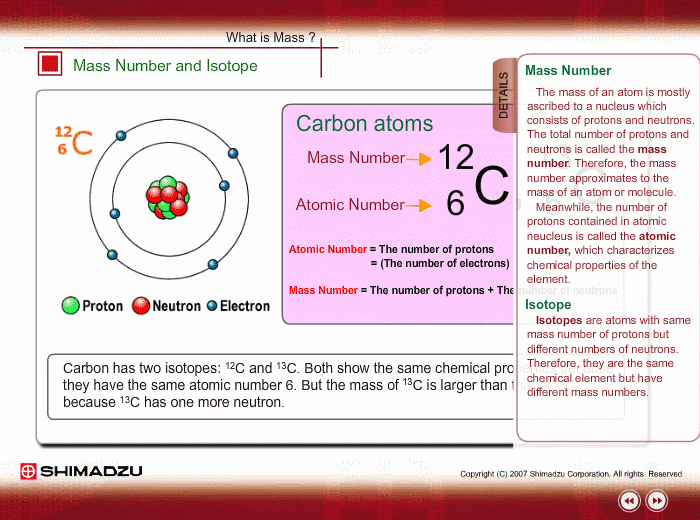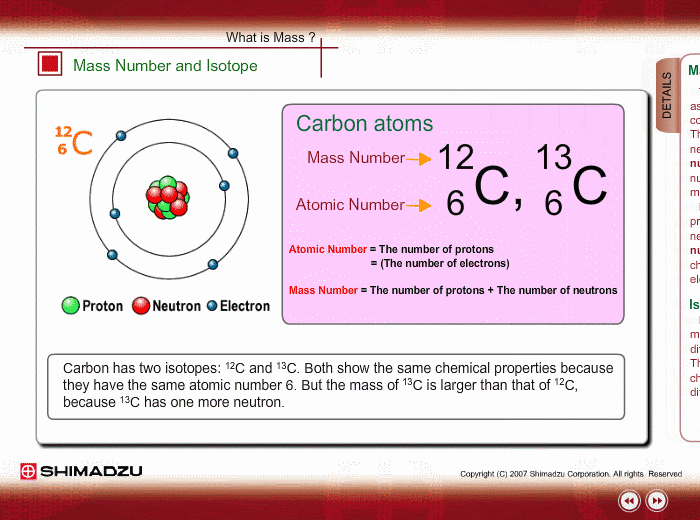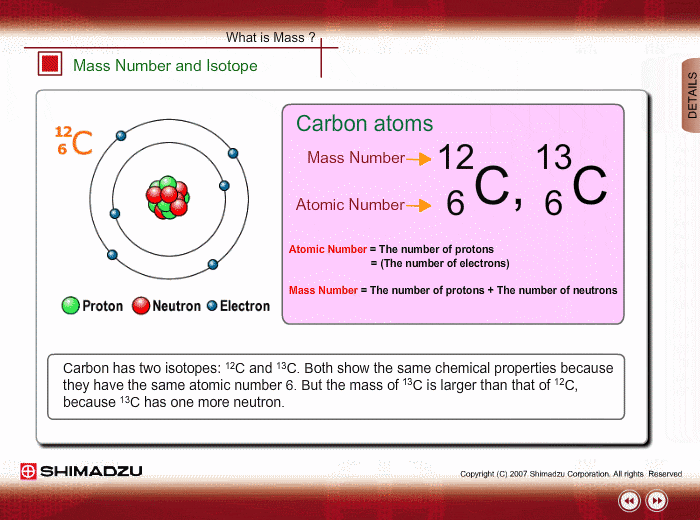
Mass Number and Isotope
Mass Number
The mass of an atom is mostly ascribed to a nucleus, which consists of protons and neutrons. The total number of protons and neutrons is called the mass number. Therefore, the mass number well approximates to the mass of an atom or molecule. Meanwhile, the number of protons is called an atomic number, which characterizes chemical features of the element.
Isotope
An isotope is a group whose constituents belong to the same chemical element but have different mass numbers. Carbon has two isotopes: 12C and 13C. Both show the same chemical features because of the same atomic number. But the mass of 13C is larger than that of 12C, because 13C has one more neutron.