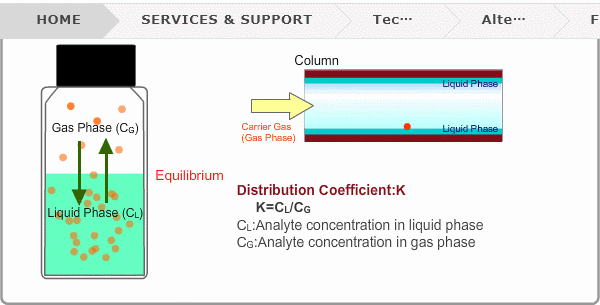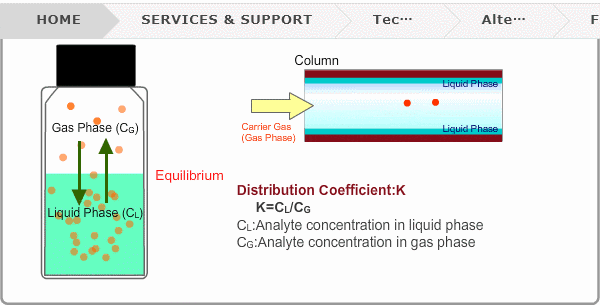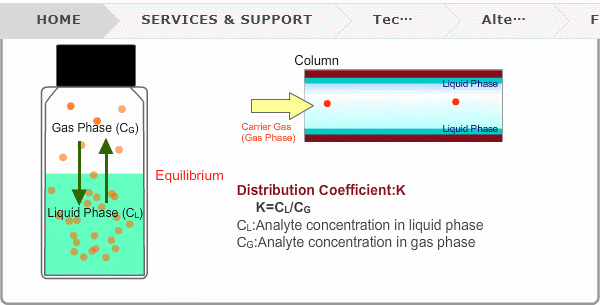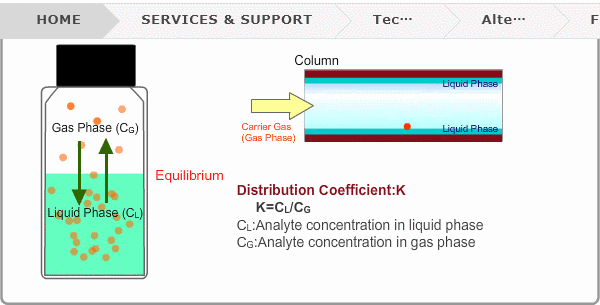
Distribution Coefficient
Roughly speaking, compounds with low boiling points tend to elute at short retention times. The distribution coefficient determines the order of elution from column. When you put liquid sample in a vial, the analyte evaporates from liquid phase (solvent) into gas phase, some of it returning to liquid phase. After a while, equilibrium will be achieved. Under this condition, the ratio K=CL/CG is constant without relation to analyte concentration. This ratio is called distribution coefficient. K depends on temperature. This notion can also be applied to column separation. There is a liquid phase (stationary phase) layer inside a column. Carrier gas corresponds to gas phase. A compound with larger K remains in the liquid phase for longer.
Next >> Retention Time Parameters


