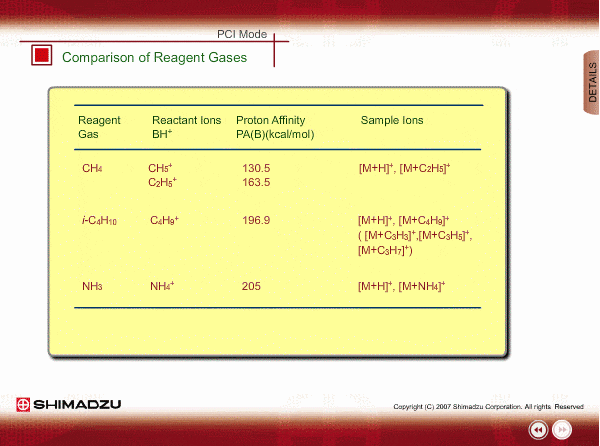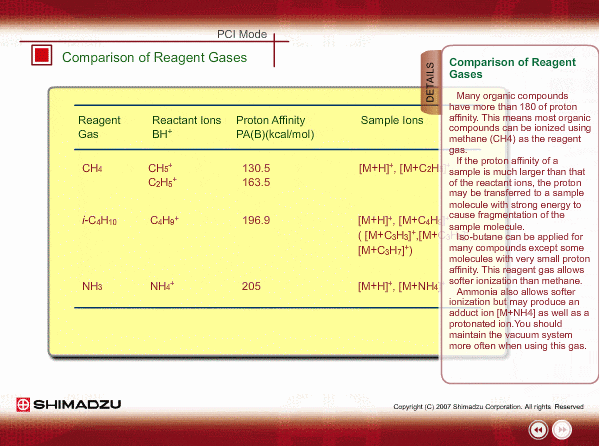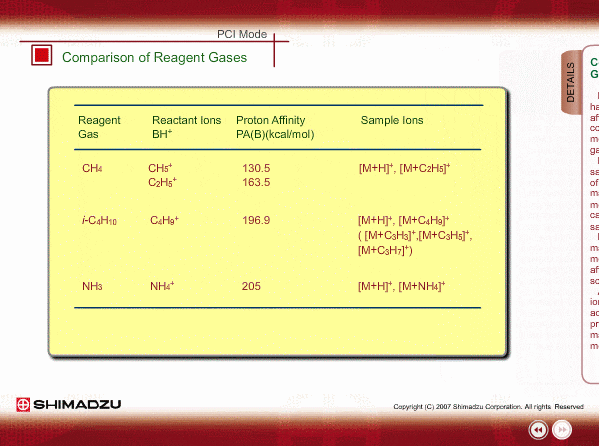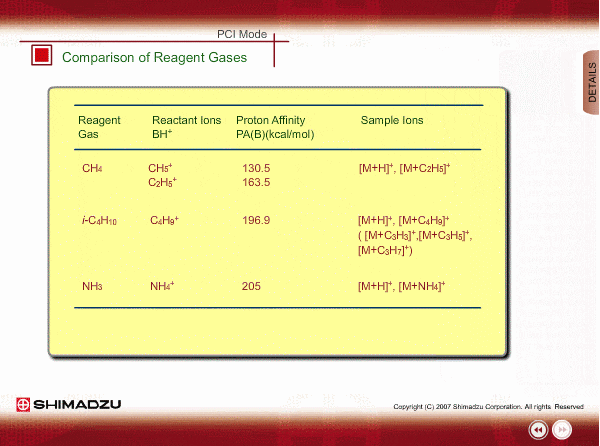
Comparison of Reagent Gasses
Many organic compounds have more than 180 of proton affinity. This means most organic compounds can be ionized as long as methane (CH4) is used as the reagent gas. If the proton affinity of a sample is much larger than that of the reactant ions, the proton may be fiercely transferred to a sample molecule with strong energy to cause fragmentation of the sample molecule.
Iso-butane can be applied for many compounds except some molecules with very small proton affinity. This reagent gas allows softer ionization than methane. Ammonia also allows softer ionization, but may produce an adduct ion [M+NH4] as well as a protonated ion. You should maintain the vacuum system more often when using this gas.
TOP >> Ionization Modes: PCI


