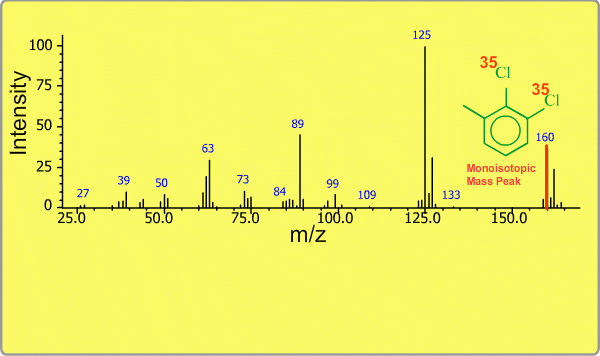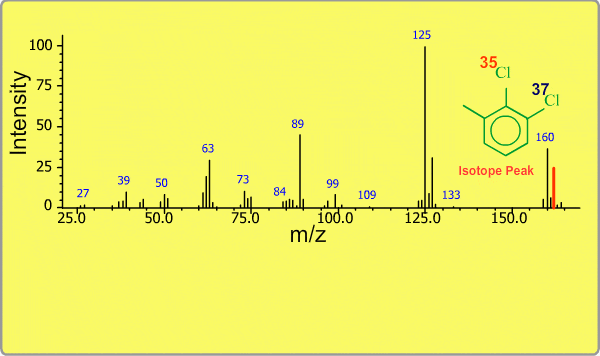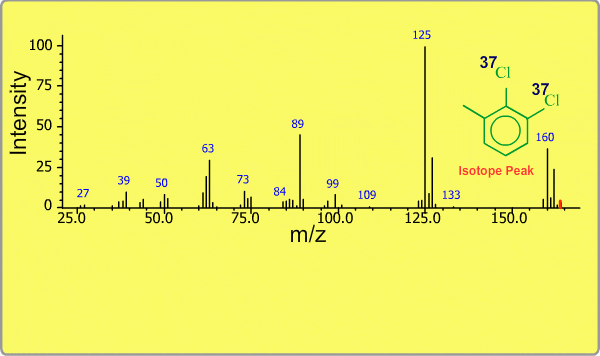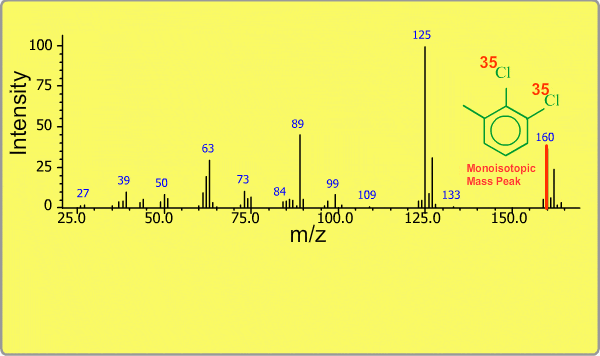
Isotope Peak
Many elements have the natural isotopes. For example, Chlorine with mass number 37 exists in addition to Chlorine 35. The presence of isotopes readily produces the isotope ions in the spectrum accompanied by a main molecular ion peak and fragment peaks. Additionally, we sometimes observe background peaks, arising from chemicals other than samples; for example, water, air, eluting materials from the column and so on.
TOP << Type of Ions


