4. Sample Injection
4.1. Sample Injection Volume
The guidelines for sample injection volumes are as follows. If the injection volume is too large, the peak shape will become deformed, or the injection port will become dirty, leading to problems.
Liquid samples become a gas at the stage of injection into the column. Liquid 1 µL → (Vaporization) → Gas, Volume: Several hundred to 1000 µL
→ In capillary analysis, it is important to determine how to inject the sample into the column efficiently.
Vaporization Volume of Liquid Samples
Vaporization Volumes of Various Solvents at an Injection Unit Temperature of 250 °C and a Pressure of 140 kPa
| Solvent | Injection Volume (µL) | |
|---|---|---|
| 1 | 2 | |
| Isooctane | 110 | 220 |
| N-hexane | 140 | 280 |
| Toluene | 170 | 340 |
| Ethyl acetate | 185 | 370 |
| Acetone | 245 | 490 |
| Dichloromethane | 285 | 570 |
| Carbon disulfide | 300 | 600 |
| Acetonitrile | 350 | 700 |
| Methanol | 450 | 900 |
| Water | 1010 | 2020 |
4.2. Sample Injection Methods
There are a variety of sample injection methods available for capillary analysis.
Hot Injection
- Split: Most of the sample is eliminated as only a portion is injected into the column.
- Splitless: Not split, but only for 1 to 2 minutes after injection
- Total volume injection (Direct injection): There is no splitting mechanism.
Cold Injection
- Cold on-column injection (OCI)
- Programmed temperature vaporization (PTV)
Reference Information
Guidelines for Applicable Samples, Injection Volumes, and Columns for Each Injection Method
This table shows the standard sample injection volumes and columns for each injection method.
| Injection Method | Hot Injection | Cold Injection | |||
|---|---|---|---|---|---|
| Split | Splitless | Total Volume | Cold On-Column Injection | Programmed Temperature Vaporization | |
| Liquid Sample | Yes | Yes | Yes | Yes | Yes |
| Gas Sample | Yes | -*1 | Yes | - | - |
| Injection Volume | Liquid sample: 2 µL max. Gas sample: 1 mL max. |
2 µL max. | Liquid sample: 2 µL max. Gas sample: 0.5 mL max. |
0.5 to 2 µL | 1 to 8 µL |
| Column | No limits on I.D. or length | I.D. 0.25 mm min. | Wide bore columns with an I.D. between 0.45 and 0.53 mm |
Wide bore columns with an I.D. of 0.53 mm × 30 m |
No limits on I.D. or length |
*1: If an additional low-temperature oven controller is attached and the column’s initial temperature can be reduced to 0 °C or less, there will be some components that can be analyzed using the splitless method.
Syringes
When injecting samples into a GC unit, a microsyringe is used for liquid samples whereas a gas-tight syringe is used for gas samples.
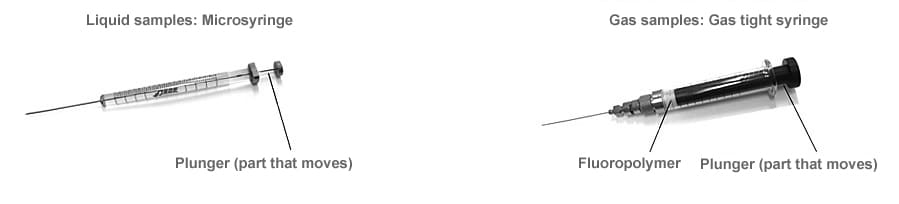
4.3. Split Injection Method
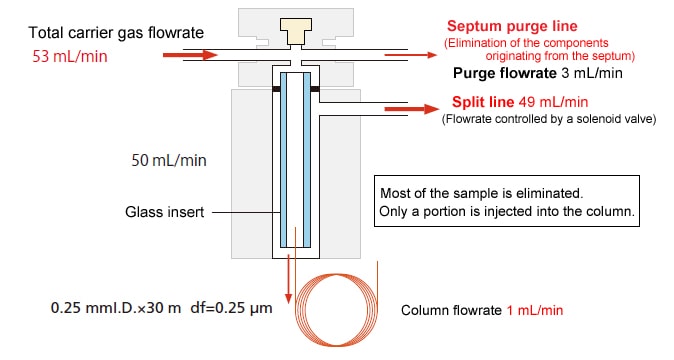
The split injection method is the most widely used injection method for capillary analysis. The optimal column flowrate (average linear velocity) for separation can be set, enabling high-separation analysis. Analysis can be performed at a wide range of concentrations, from medium to high, and it is suitable for relatively high-concentration samples.
Some of the carrier gas flows into the septum purge line to remove the components emerging from the bottom of the septum. The remainder flows into the insert within the sample injection unit. A portion branches off to the column, while the remainder branches off to the split line (split). With this injection method, most of the sample is eliminated. Only a portion is injected into the column. For this reason, it is not suitable for trace analysis.
Schematic Diagram
Example with a Split Ratio of 49
- If the split ratio is small, the peak may inadvertently broaden at the injection port, reducing the separation
- Only a portion of the injected sample is injected into the column, so the sample vaporized within the insert must be uniform.
→ Be sure to place silica wool within the glass insert. Depending on the sample, change the amount and positioning of the wool, or the filler within the insert.
4.4. Splitless Injection Method
This method is used for low-concentration samples that require higher sensitivity than what the split method provides.
In the context of capillary analysis, this is a relatively limited analysis method in terms of the applicable components, so it is mainly used for trace analysis (several tens of ppm or less).
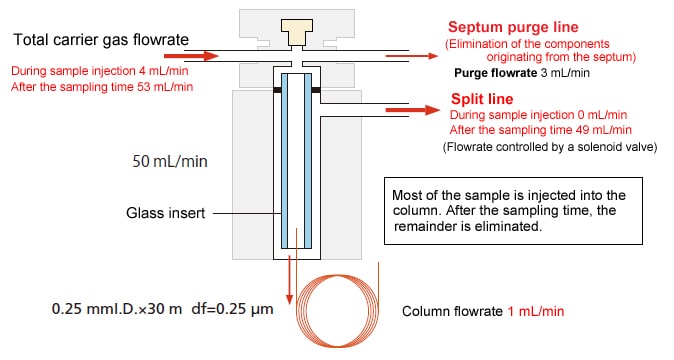
The time from when the sample is injected until the split line is opened is called the sampling time (splitless time). Samples that are not injected within this time are eliminated.
The volume of sample injected into the column is determined by the formula: Column flowrate × Time. This is unrelated to the split ratio.
Peak broadening can be avoided by keeping the column temperature lower than the boiling point of the sample during the sampling time.
Schematic Diagram
Example with a Split Ratio of 49
- A septum purge line is required in order to reduce the impact of sample retention and compounds originating from the septum.
- Programmed temperature analysis is essential.
- The method is not suitable for gas samples, low boiling point solvent samples, and components eluted in the vicinity of solvents.
- The method is difficult to apply to components that elute faster than the solvent.
4.5. Total Volume Injection Method
With the total volume injection method, the entire sample can be injected into the column.
In this analysis method, an injection port is connected to a wide bore column with an I.D. of at least 0.45 mm. As in packed analysis, almost all of the injected sample is injected into the column. There are two methods. In one, analysis is performed by attaching a WBC (Wide Bore Capillary Column) attachment to the sample injection unit used for the packed column. In the other, analysis is performed with an injection port for total volume injections.
Schematic Diagram
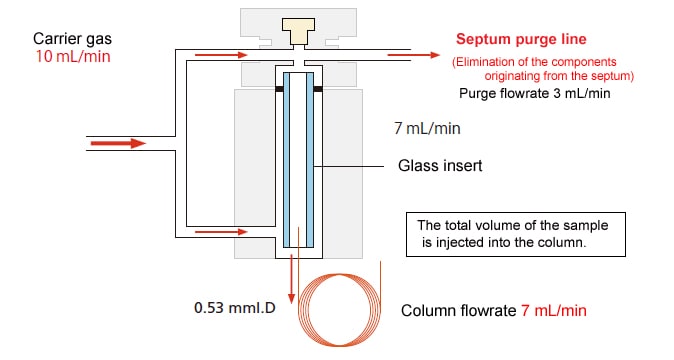
- If you are using the WBC attachment, set the carrier gas flowrate to 8 to 10 mL/min. If the column flowrate is too low, it will take too long for the injected sample to move into the column, which might reduce the separation.
4.6. Cold On-Column Injection (OCI) Method
This is a highly precise analysis method featuring surface area reproducibility. This is the GC analysis method with the least pyrolysis of the sample. It is suitable for samples with low concentrations of measurement components. (It is suitable for samples with concentrations of approximately 200 ppm or less per component targeted for analysis.)
In this sample injection method, the sample solution is injected as is by inserting the tip of the microsyringe needle directly into the tip of the capillary column, while maintaining the injection port temperature below the boiling point of the sample solvent. After this, the injected components can be directly and gently vaporized within the capillary column by increasing the temperature of the injection port and the column. The tip of the capillary column is equivalent to an injection unit.
Schematic Diagram
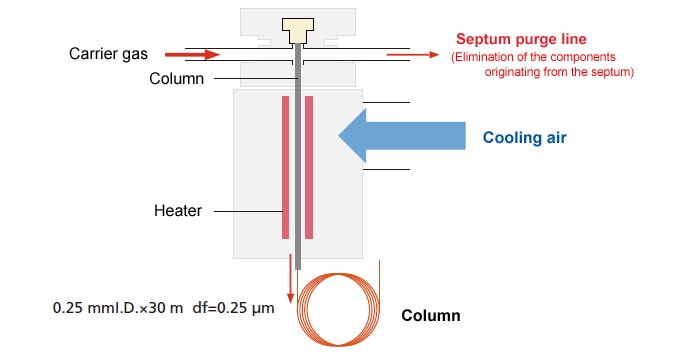
- The sample is injected directly into the column, so the column tends to become dirty, and periodic maintenance is required.
4.7. Programmed Temperature Vaporization (PTV)
In this injection method, when the sample is injected, the injection port is set to below the boiling point of the injection sample solvent. After the sample is injected, the injection port is heated rapidly, causing the injected sample to vaporize. Changes in composition (discrimination) due to heating of components remaining in the syringe needle tip are minimal, so this is suitable for the analysis of compounds that are thermally unstable (prone to degradation).
Unlike OCI analysis, a glass insert is used, and the method can be used for both split and splitless analysis, enabling support for low and high-concentration samples. In this analysis method, the column does not become very dirty even when analyzing samples containing many relatively nonvolatile components. Large volume injections (LVI) can be performed by using a GC unit equipped with an electronic flow controller (AFC) to control the carrier gas flowrate.
Schematic Diagram
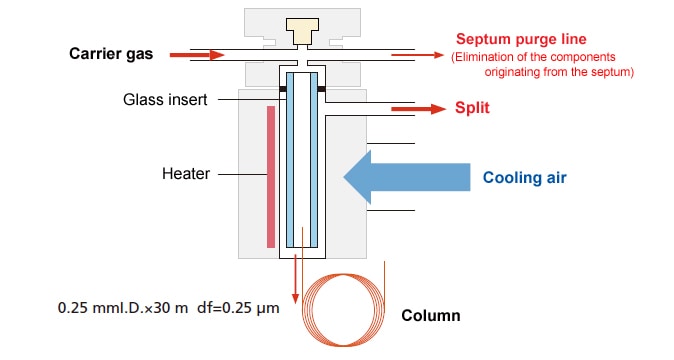
- Programmed temperature analysis is required.
- The initial temperature of the column should be set to below the boiling point of the injected sample solvent (approximately the same as the initial temperature for PTV).


