6. Detector
6.1. Types of Detector
The detectors that can be used with Shimadzu gas chromatographs are shown below. They are broadly divided into general-purpose detectors and selective, high-sensitivity detectors. General-purpose detectors can analyze a wide range of compounds, of which the flame ionization detector (FID) is the most common because it can analyze almost all organic compounds. In contrast, selective, high-sensitivity detectors are only capable of detecting specific types of compounds selectively and with high sensitivity.
| Detector | Detectable Compound | Detection Limit* |
|---|---|---|
| General-Purpose Detectors | ||
| Flame ionization detector (FID) | Organic compounds (other than formaldehyde and formic acid) | 0.1 ppm (0.1 ng) |
| Thermal conductivity detector (TCD) | All compounds other than the carrier gas | 10 ppm (10 ng) |
| Barrier discharge ionization detector (BID) | All compounds other than He and Ne | 0.05 ppm (0.05 ng) |
| Selective, High-Sensitivity Detectors | ||
| Electron capture detector (ECD) | Organic halogen compounds Organic metal compounds |
0.1 ppb (0.1 pg) |
| Flame thermionic detector (FTD) | Organic nitrogen compounds Inorganic and organic phosphorus compounds |
1 ppb (1 pg) 0.1 ppb (0.1 pg) |
| Flame photometric detector (FPD) | Inorganic and organic sulfur compounds Inorganic and organic phosphorus compounds Organic tin compounds |
10 ppb (10 pg) |
| Sulfur chemiluminescence detector (SCD) | Inorganic and organic sulfur compounds | 1ppb(0.1pg) |
*The detection limits are approximations. Actual values will vary depending on the compound structure and analytical conditions.
6.2. Detector Gas and Makeup Gas
Each detector requires gas, called the detector gas, based on its principle of detection. For example, the flame ionization detector (FID) uses a hydrogen flame so it requires hydrogen and air.
Analysis using a capillary column can also require a makeup gas added just before the detector to act as an auxiliary gas and ensure the detector receives a rapid supply of compounds. Makeup gas reduces the effects of increasing and decreasing column flowrates on detector sensitivity by increasing the sample transfer speed inside the detector and preventing peak broadening.
| Detector | Detector Gas | Makeup Gas (Capillary) |
|---|---|---|
| FID | H2 and Air | He or N2 |
| TCD | Unnecessary | He or Ar or N2 or H2 ,etc. |
| BID | He | None |
| ECD | Mainly N2 (The combination of gases varies by equipment model.) | |
| FTD | H2 and Air | He |
| FPD | H2 and Air | None (required in some models) |
| SCD | H2 and O2 | N2 |
6.3. General-Purpose Detectors
6.3.1. Flame Ionization Detectors (FID)
The FID is the most common detector used in gas chromatography. The FID is sensitive to, and capable of detecting, compounds that contain carbon atoms (C), which accounts for almost all organic compounds. However, the FID is not sensitive to carbon atoms with a double bond to oxygen, such as in carbonyl groups and carboxyl groups (CO, CO2, HCHO, HCOOH, CS2, CCl4, etc.).
|
< Main Applications >
|

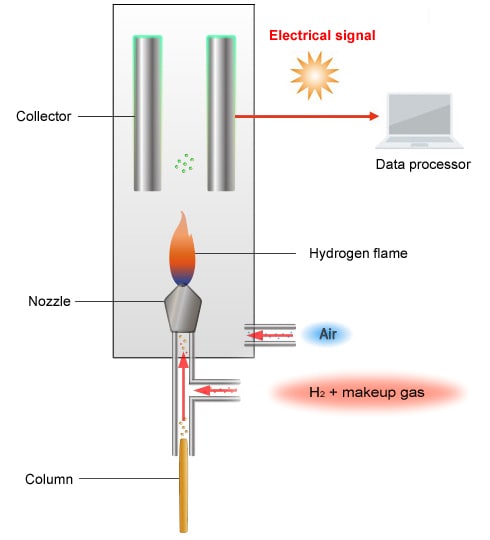
Schematic Diagram of the FID
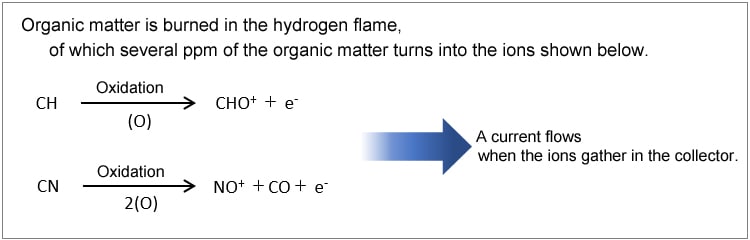
The FID creates a hydrogen flame by burning air and hydrogen supplied from below. The carbon in a sample carried into the detector on carrier gas is oxidized by the hydrogen flame, which causes an ionization reaction. The ions formed are attracted by a collector electrode to an electrostatic field, where the components are detected.
6.3.2. Thermal Conductivity Detectors (TCD)
The TCD can detect all compounds other than the carrier gas. The TCD is mainly used to detect inorganic gas and components that the FID is not sensitive to.
Helium is commonly used as a carrier gas. (N2 and Ar are used to analyze He and H2.)
|
< Main Applications >
|
Schematic Diagram of the TCD
The principle of detection used by the TCD is as follows. The TCD detects target components by reading the change in filament temperature caused by the difference in thermal conductivity between the carrier gas and target components.
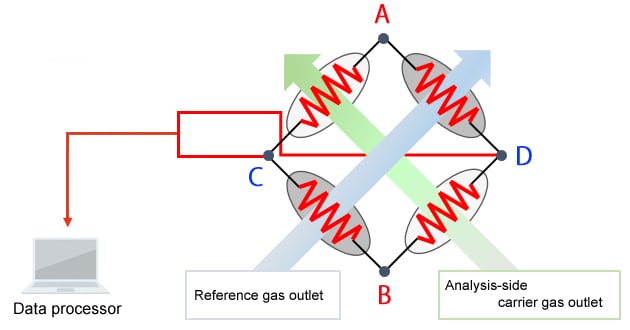
A direct voltage is applied between A and B.
• When only the carrier gas is flowing at a constant flowrate
-Each filament maintains a constant temperature and a constant voltage is produced between C and D.
• Components are eluted from an analysis-side column.
-A change in filament temperature occurs, which
-Changes the resistance value, and
-Changes the voltage between C and D
Thermal Conductivity Coefficients (10-6 cal/s ·cm ·°C)
| Component | Thermal Conductivity |
|---|---|
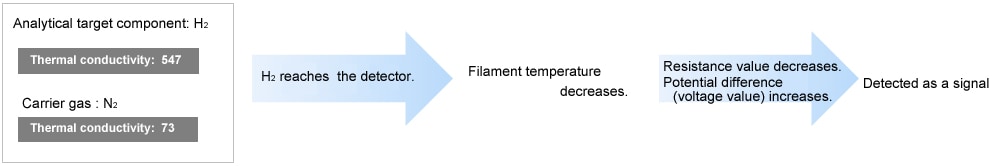
| H2 | 547 (extremely high) |
| He | 408 (extremely high) |
| Ethane | 77 |
|
O2
|
76
|
| N2 | 73 |
| H2O | 60 |
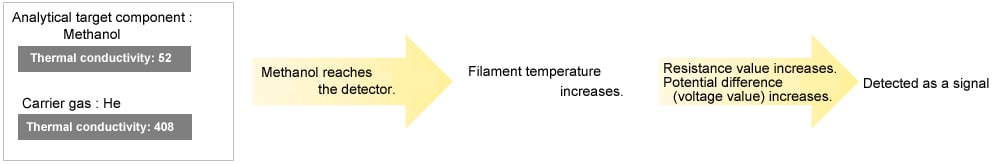
| Ar,methanol | 52 |
| Methanol | 40 |
| Chloroform | 24 |
TCD Analysis Example
When the thermal conductivity of the analytical target component is lower than the carrier gas, the TCD reads an elevation in filament temperature. Conversely, when the thermal conductivity of the analytical target component is higher than the carrier gas, the TCD reads a decrease in filament temperature.
When the Thermal Conductivity of the Analytical Target Component is Lower than the Carrier Gas
Selection by Analytical Objective
Related Application News
6.3.3. Barrier Discharge Ionization Detectors (BID)
The BID is Shimadzu’s proprietary detector that can detect all inorganic and organic compounds other than He and Ne. The BID is also capable of detecting trace amounts of impurities at the ppm level that the TCD failed to detect during an inorganic gas analysis.
|
< Main Applications >
|
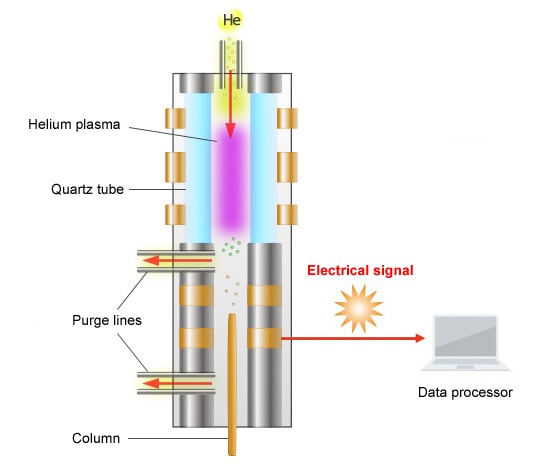
Schematic Diagram of the BID
The principle of detection used by the BID is as follows.
The BID generates a stable He plasma, uses the energy emitted by the excited He to ionize compounds, then attracts these ions to a collector. The He plasma energy emitted is extremely high and capable of ionizing all compounds other than He, which is used to create the plasma, and Ne, which has extremely high ionization energy. As a result, the BID can detect any compound, in principle, other than He and Ne.
Principle of Ionization
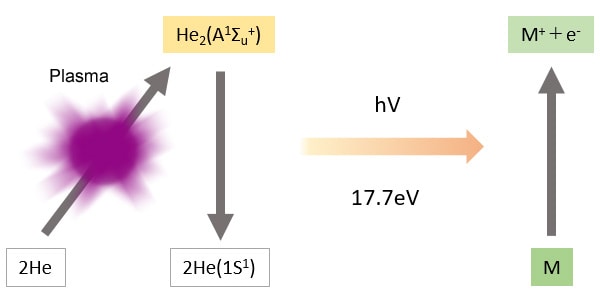
Compounds eluted from the column are ionized by light energy from the plasma.
-Ions are attracted to the collection electrode and output as peaks.
The light energy from the He plasma is 17.7 eV (electron volts), which is extremely high.
-The BID is capable of high-sensitivity detection of all compounds other than the plasma gas He, and Ne, which has a higher ionization energy than He.
Related Application News
6.4. Selective, High-Sensitivity Detectors
6.4.1. Electron Capture Detectors (ECD)
The ECD is a selective, high-sensitivity detector for electrophilic compounds. The ECD is capable of detecting organic halogen compounds, organic metal compounds, diketone compounds, etc. Because the ECD is fitted with a radioactive isotope, installation requires a notice of use be sent to the Japanese Ministry of Education, Culture, Sports, Science and Technology.
|
< Main Applications >
|
Schematic Diagram of the ECD
The principle of detection used by the ECD is as follows. The ECD detects ions by reading the change in voltage value that maintains a constant ion current gathered at the collector.
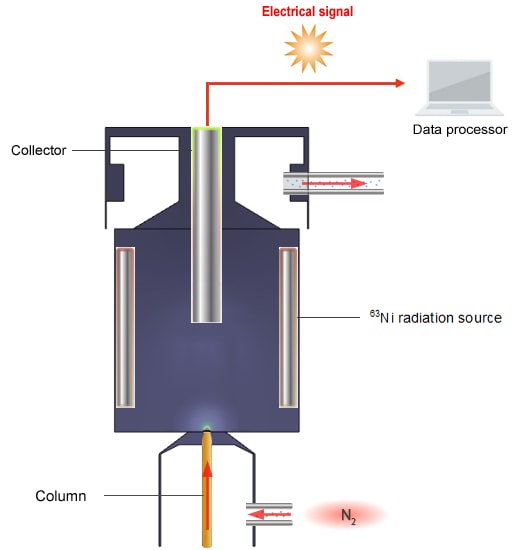
N2, which is used as the carrier gas, is ionized by β waves emitted from the 63Ni radiation source.
N2 → N2+ + e-
A current flows when the ions gather in the collector.
When an electrophilic compound is placed in this equation,
PCB + e- → PCB-
PCB- is much larger and heavier than e- and so takes more time to reach the collector.
-A higher voltage is needed for a constant ion current to flow.
6.4.2. Flame Thermionic Detectors (FTD)
The FTD is a selective, high-sensitivity detector for organic nitrogen compounds and inorganic and organic phosphorus compounds. (The selectivity of the FTD for phosphorus compounds is not as good as the FPD.) The FTD does not react to inorganic nitrogen compounds.
|
< Main Applications >
|
Schematic Diagram of the FTD
The principle of detection used by the FTD is as follows. The FTD detects ions by reading the change in ion current gathered at the collector.
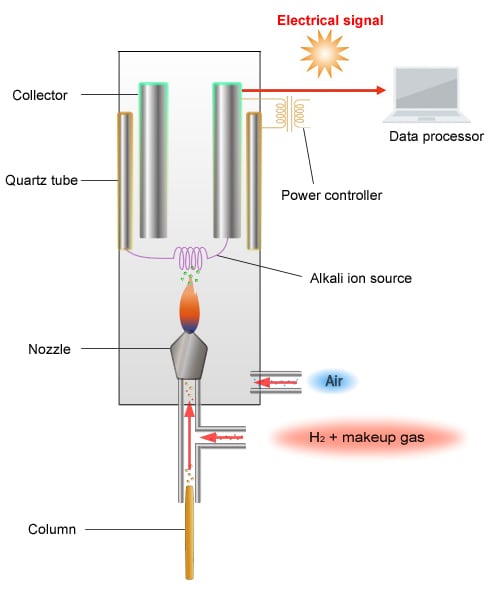
When a current is passed through the platinum coil with an alkali source attached to the coil (rubidium salt), the coil increases in temperature, which creates plasma around the alkali source.
Rubidium radicals (Rb*) are generated within this plasma.
-Capable of oxidizing CN and organic phosphorus compounds
-PO2 reacts with Rb* as shown below, creating ions.
CN + Rb* → CN- + Rb+
PO2 + Rb* → PO2- + Rb+
A current flows when ions gather in the collector.
6.4.3. Flame Photometric Detectors (FPD)
The FPD is a selective, high-sensitivity detector for phosphorus (P) compounds, sulfur (S) compounds, and organic tin (Sn) compounds. The FPD is highly selective as it detects element-specific light emitted within a hydrogen flame.
|
< Main Applications >
|
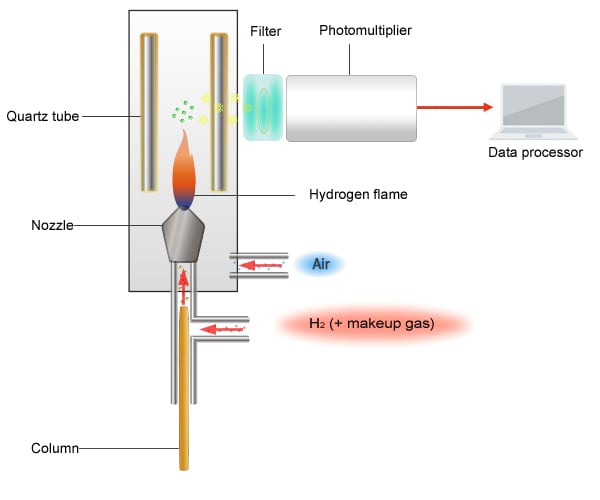
Schematic Diagram of the FPD
The principle of detection used by the FPD is as follows.
Sulfur compounds, phosphorus compounds, and organic tin compounds each emit light at unique wavelengths when burned. By passing light through a filter, only light of these unique wavelengths reaches a photomultiplier tube. The photomultiplier tube then converts the detected light intensity into an electrical signal.
6.4.4. Sulfur Chemiluminescence Detectors (SCD)
The SCD is a selective, high-sensitivity detector for sulfur (S) compounds. The SCD is highly sensitive and capable of detecting infinitesimal amounts of sulfur compounds.
Compared to the FPD, which is similarly capable of selective detection of sulfur compounds, the SCD is around one order of magnitude more sensitive and exhibits a proportional linear relationship between the SCD sensitivity and the sample concentration. (This relationship is quadratic for the FPD.) The SCD also exhibits equimolar sensitivity and measures sulfur compounds with the same relative sensitivity regardless of compound structure.
This characteristic of the SCD allows the use of calibration curves for other compounds to determine an approximate concentration of a target compound, even when no standard sample is available.
The SCD also differs substantially from other detectors in that a low-pressure environment is maintained inside the SCD.
|
< Main Applications >
|
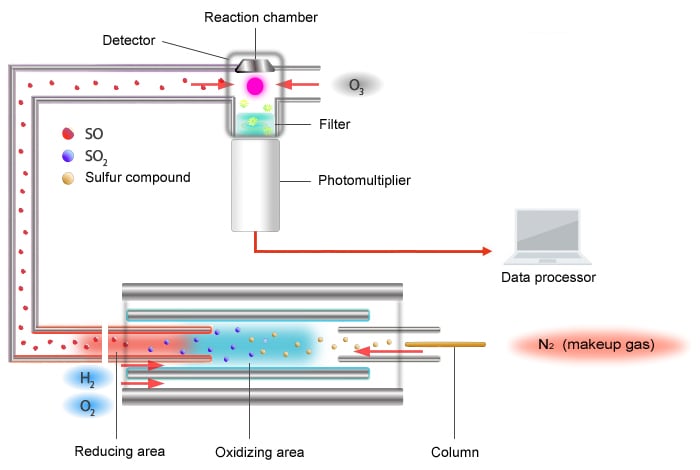
Schematic Diagram of the SCD
The principle of detection used by the SCD is as follows.
The sulfur chemiluminescence detector (SCD) uses the chemiluminescence reaction caused by ozone oxidation.
Sulfur compounds are converted to an X-S chemical species (mainly SO) that is capable of exhibiting chemiluminescence inside an extremely high temperature (around 1000 °C) oxidative-reductive furnace. The X-S chemical species is carried to the detector area where ozone converts it into an excited-state SO2* (radical). The SO2* emits light upon returning to its base state, and the SCD detects the sulfur component by measuring this light with a photomultiplier tube.