Principle of Separation in HPLC
-
There are 3 factors in the column: Compounds, Mobile Phase, and Stationary Phase. Each definition is shown as follows;
- Compounds : Solutes in the sample solution
- Mobile Phase : Solution delivered using solvent delivery pumps
- Stationary Phase : Functional groups chemically modified in spherical particles packed in the columnFig.1 schematically shows the relationship between the sample compounds and the stationary and mobile phases.
Compounds that interact more strongly with the mobile phase elute more faster from the column.
In contrast, compounds that interact more strongly with the stationary phase remain in the column longer. -
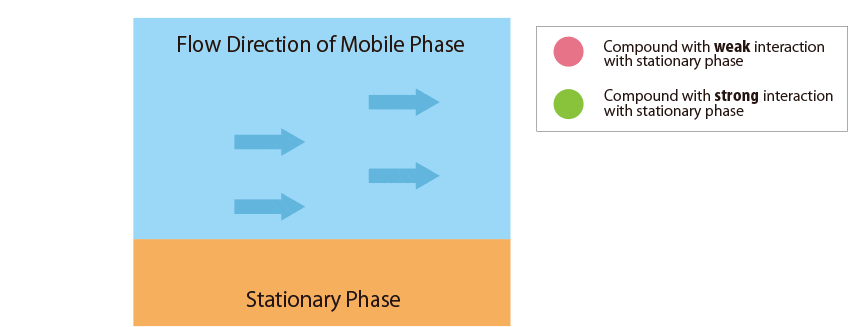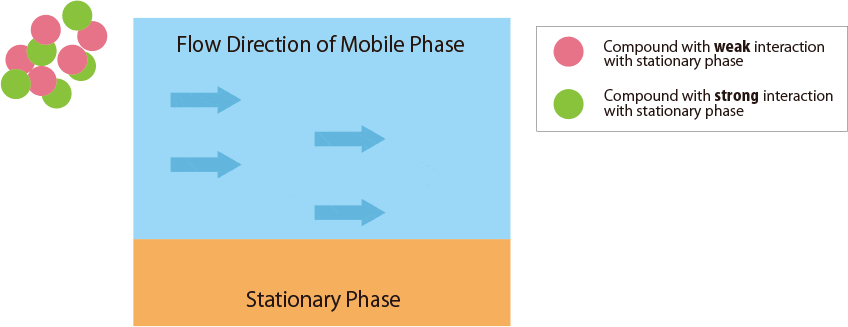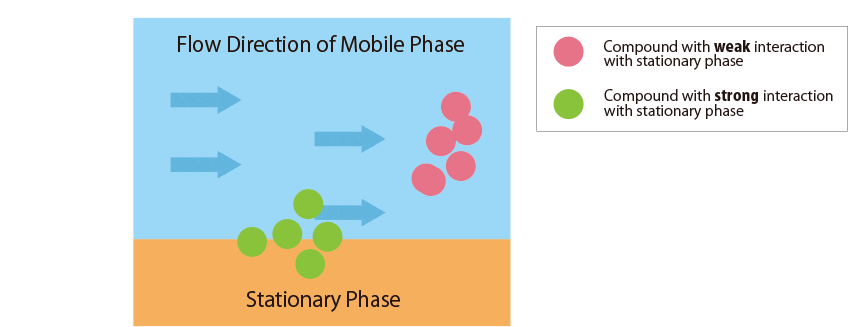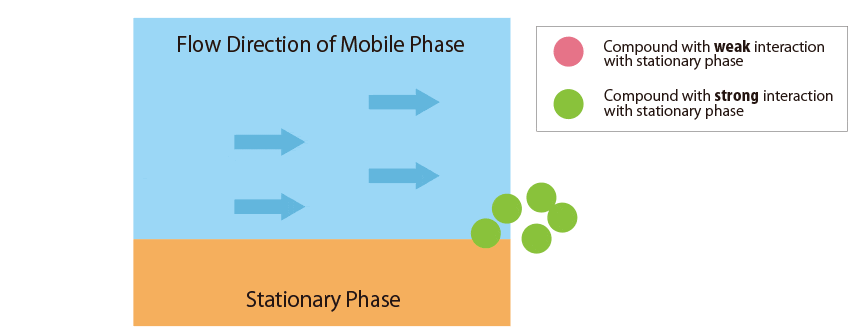
Fig.1 Mechanism of separation in the HPLC column
"Interaction" refers to the chemical attraction between molecules. The selection of the interaction type suitable for the property of the target compound is necessary for the optimization of the separation method. The type of interaction is related to the separation mode. Table 1 shows the list of general HPLC separation modes and basic principle.
Table 1 List of general HPLC separation modes and basic principle
| Separation Mode | Interaction | Components |
|---|---|---|
| Reversed phase chromatography (RP) | Hydrophobicity(Low Polarity) | Small molecule pharmaceuticals, Vitamins, etc. |
| Normal phase chromatography (NP) | Hydrophilicity (High Polarity) | Saccharides, Nuclear acids, etc. |
| Ion exchange chromatography (IEX) | Electrostaticity | Inorganic ions, Amino acids, Protein, etc. |
| Size exclusion chromatography (SEC) | Molecular size | Synthetic polymer, Biopolymer, Polysaccharide, etc. |


