Fundamentals of Liquid Chromatography Mass Spectrometry (LCMS)
Applications, and Analysis Solutions
There are different techniques of liquid chromatography (LC) available and the principles of retention and separation of each LC technique differ. The table below presents a list of the common LC techniques and their separation mechanism. With current advancements in LC, it has evolved into technologies of smaller particle sizes and higher pressure that are more efficient and of higher speed, sensitivity, and resolution such as high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC).
| Type of LC Techniques | Separation Mechanism | Commonly used for |
|---|---|---|
| Reversed-Phase LC (RPLC) | A non-polar stationary phase and a polar mobile phase are used for RPLC. Based on the "like attracts like" principle, the sample is separated based on the molecule's polarity preference to either the polar mobile phase or the non-polar stationary phase. For example, the non-polar molecules will prefer to retain in the non-polar stationary phase rather than the polar mobile phase. As a result, it gets eluted later compared to the polar molecules. | Low molecular weight (MW) compounds |
| Normal Phase LC (NPLC) | NPLC works entirely opposite to RPLC. In NPLC, a polar stationary phase and a non-polar mobile phase is used. Polar molecules are strongly retained by the stationary phase as compared to the non-polar molecules. As a result, the non-polar molecules elute first. | Steroid hormones, phospholipids, saccharides and tocopherols |
| Hydrophilic Interaction Liquid Chromatography (HILIC) | HILIC works on the same principle as NPLC. The main difference is that water is added in the organic mobile phase to effectively separate and elute the strongly-retained polar molecules. | Polar compounds |
| Ion Exchange Chromatography (IEC) | IEC retains and separates charged species (ions) based on electrostatic affinity of the analyte for the stationary phase containing a functional group of an opposite charge. Differential elution is induced either by changing the pH of the mobile phase to neutralize the analyte, or by increasing the ionic strength (salt concentration) to competitively displace the analyte. | Proteins, amino acids, nucleotides, and inorganic ions |
| Size Exclusion Chromatography (SEC) | The SEC column used is filled with porous particles. When samples of various sizes flow into the column, smaller molecules migrate more slowly because they penetrate deep into the pores, whereas large molecules flow quickly as they do not enter the pores as much. As a result, larger molecules elute from the column sooner, which effectively sorts the samples by molecular size. | Proteins and synthetic high polymers |
Upon separation by LC, the components can be detected using optical properties such as ultraviolet-visible (UV-Vis), fluorescence, refractive index, evaporative light scattering or electrical conductivity based on the analytes' properties.
In the case of liquid chromatography mass spectrometry (LCMS), MS offers a highly sensitive detection technique that ionizes the sample components, separates the resulting ions in vacuum based on their mass-to-charge ratios (m/z) and measures the intensity of each ion. A mass spectrum plots the relative ion intensities against the m/z values, and a series of mass spectra are generated at each time point. This information indicates the concentration level of ions that have a given mass and is extremely valuable for the unique identification of molecules, also known as qualitative analysis. Moreover, MS provides added specificity and sensitivity, and the convenience of simultaneous multicomponent analysis.
With the coupling of LC with MS, the mass spectra obtained from these measurements provide molecular mass and structural information for eluted components, which supplement the qualitative information based on retention times obtained using other LC detectors. Therefore, LCMS combines the outstanding separation resolution of LC with the excellent qualitative capabilities for application needs.
Comparison of LCMS and Other Techniques
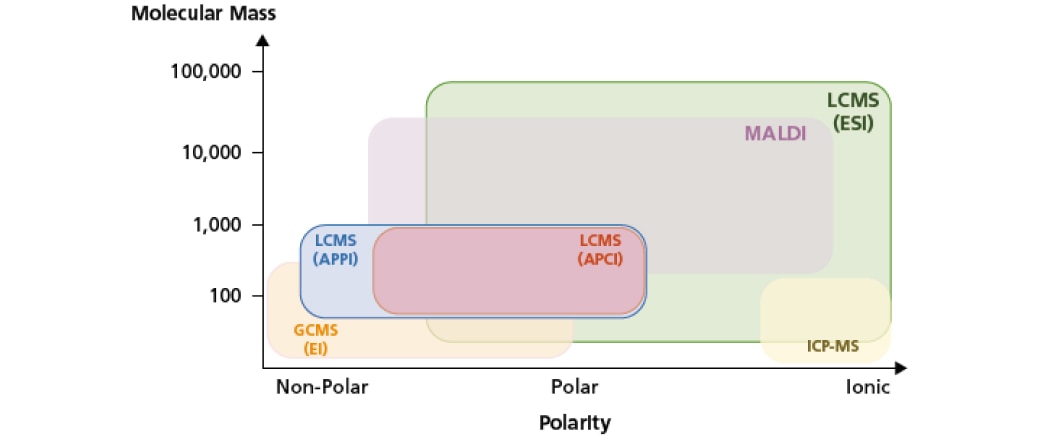
Apart from LCMS, there are various ionization techniques and analytical instruments for chemical analysis such as Inductively Coupled Plasma MS (ICP-MS), Matrix-Assisted Laser Desorption/Ionization (MALDI-MS) and electron ionization (EI) with gas chromatography MS (GCMS). MS was first introduced and used as a detector for GC systems in the late 1960s and their advantages have been widely recognized.
LCMS was later made possible in the mid 1970s with the introduction of atmospheric pressure ionization (API). With the use of API, the LC eluent vaporizes and ionizes to form gaseous ions, which are subsequently introduced into the MS. API is a type of soft ionization technique and examples include atmospheric pressure photoionization (APPI), atmospheric pressure chemical ionization (APCI) and the commonly used electrospray ionization (ESI).
Unlike GCMS, one of the advantages of LCMS is its broad applicability to a wide range of compounds. Once the sample is dissolved in a mobile phase, LCMS can analyze even the least volatile or thermally unstable compounds that are difficult to analyze using GCMS.
Other ionization techniques such as ICP and MALDI were later introduced in the mid 1980s. ICP-MS is frequently applied for elemental analysis while MALDI, a soft ionization technique, is commonly used for large molecules analysis (e.g. proteins, peptides and polymers). In summary, with the introduction of API techniques, LCMS provides broad applicability for an extensive range of substances (e.g. polar and nonpolar) and offers high selectivity.
Applications of Liquid Chromatography Mass Spectrometry (LCMS)
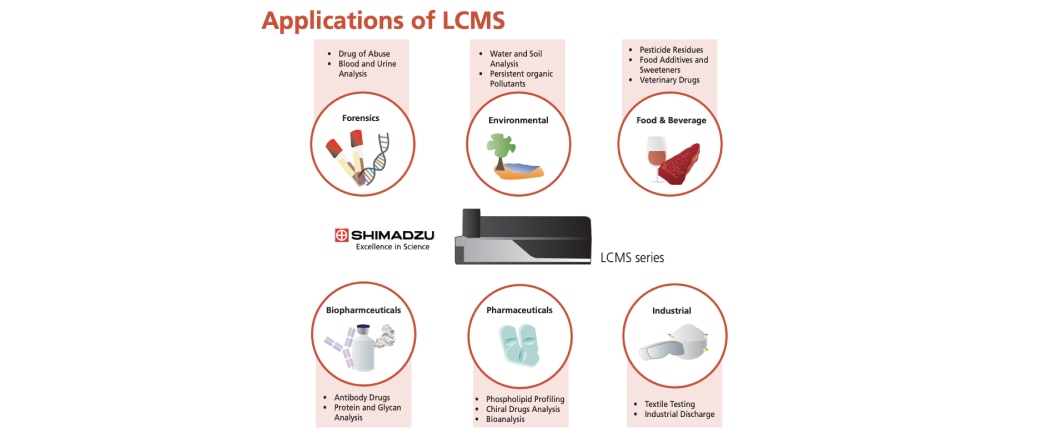
With its superior qualitative and quantitative capabilities and robustness, LCMS is commonly used to meet the rigorous demands of the analytical market and industries. LCMS is applied in many industries such as pharmaceuticals, biopharmaceuticals, forensic, industrial, food and environmental sectors.
For clinical research, the analysis of drugs, vitamins and minerals in whole blood, plasma, serum and urine is conducted routinely using LCMS. It is also applied in metabolomics, proteomics and genomics study. The use of LCMS in the biopharmaceutical discipline has enabled the bioanalysis and characterization of antibody drugs.
In the environmental field, LCMS is widely utilized for the qualitative and quantitative determination of known pollutants (e.g. pesticides, bacteria, pharmaceuticals and personal care products) and trace-level emerging contaminants.
Food safety and development have also adopted the use of LCMS in their product quality control such as the quantitation of residual veterinary drugs, food additives and the composition analysis of supplements and organic foods.
With high sensitivity, high detection selectivity, and high qualitative capability, MS brings about the flexibility of simultaneous multi-component analysis, improved productivity, and efficiency to HPLC analyses in these applications.
Analysis Solutions Using LCMS
Explore our Technology Solutions to discover analysis solutions using LCMS. From quantifying pesticides in fruits to rapid detection of illicit drugs, natural product analysis, and a guide to streamlining LCMS workflow, discover how Shimadzu’s triple quadrupole mass spectrometers offer fast, robust, and high-throughput capabilities to meet analysis needs across diverse applications.
Get to access our Technology Brief to explore the latest technological advances in the instruments’ capabilities in powering the analysis. Learn how you can speed up LCMS analysis with Shimadzu’s proprietary UFMSTM technology, method packages, and so much more in our Resource Library.
For more, discover our Digital Classrooms and our instruments here.
Accelerate your analyses with our solutions today.


