Automate AQbD Method Development With LabSolutions MD

Analytical Quality by Design (AQbD) is recommended by the International Council for Harmonization (ICH) to emphasize rigorous scientific foundation and risk assessment in the development of analytical methods. It comprises a comprehensive evaluation of the analytical conditions without relying on an operator’s experience or intuition. AQbD thus increases reliability and enables the efficient development of accurate, robust analytical methods. Using this systematic approach, analysts gain an understanding of the separation, which also enables informed decisions in troubleshooting and continuous improvement of test methods.
The method development workflow according to AQbD principles is as follows:
Screening
- Column and mobile phase screening
- Experiments to evaluate the most suitable combination of stationary and mobile phase
Optimization
- Computer-aided method optimization
- Optimization of gradient conditions, temperature, flow rate, etc. using a design space
Robustness
- Confirmation of robustness
- Evaluation of the effect of small variations in methods parameters on the chromatographic results
Introducing the Capabilities of LabSolutions MD

LabSolutions MD stands as Shimadzu’s automated solution for optimizing method development using the AQbD approach. This software streamlines the creation of robust analysis methods by configuring mobile phases, columns, and other parameters using an analysis function that automatically generates analysis schedules with the experimental design method and a data analysis function that plots a design space and predicted chromatogram.
Keep a copy of our brochure on hand for quick reference to essential information about LabSolutions MD, whenever you need it.
Benefits of LabSolutions MD For Method Development
LabSolutions MD facilitates all steps in the screening, optimization, and validation phases of the method development workflow.
- Automatic Screening of Mobile Phases and Columns: Mobile phases and columns are automatically switched to find the optimal combination without human intervention.
- Automatic Optimization of Gradient Conditions: Gradient conditions that satisfy criteria are automatically explored.
- Easy-to-Use Software: Easy creation of analysis schedules with various conditions. Chromatograms obtained can be ranked based on separation performance.
Workflow of AQbD Approach for Method Development
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) suggests the AQbD approach for method development. It is recommended to acquire data by conducting efficient experiments, such as with the use of experimental design, verifying the parameters that have a large effect on analytical results, and then building a design space to understand the effective domain of the parameters with respect to the analysis results. This risk-based approach ensures the development of robust, low-risk methods without relying on user experience.
Screening Phase: Initial Screening
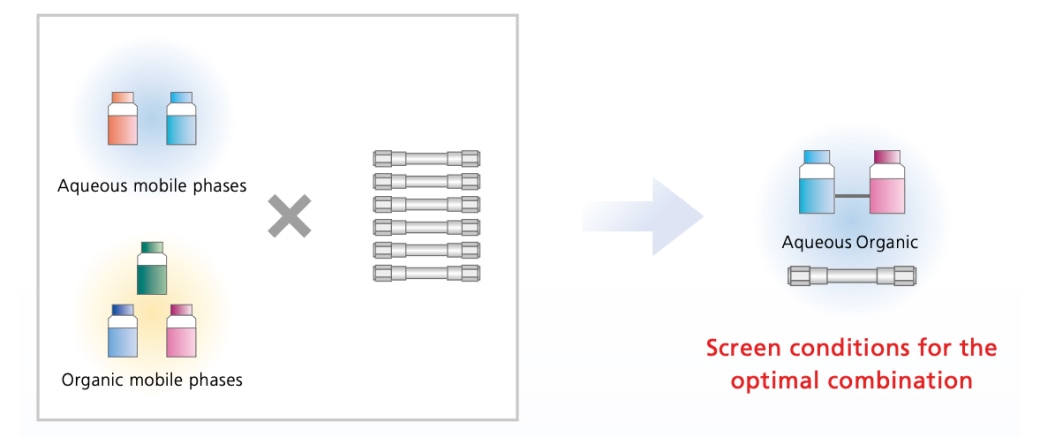
Initial screening is based on parameters that have a major effect on peak retention time and separation, such as the pH of aqueous mobile phases, the mixture ratio of organic mobile phases, and the type of column.
Using LabSolutions MD, it is possible to use two types of aqueous mobile phases, three types of organic mobile phases, and six types of columns to acquire a total of 36 data points (full factorial design) for screening mobile phase and column conditions.
Optimization Phase: Optimization
With the analytical conditions determined by initial screening results as a starting point, optimal setting levels are verified for other parameters, such as pump gradient and column oven temperature conditions.
Because screening generates as many chromatograms as the number of conditions considered, they must be evaluated to determine which is optimal. If all the chromatograms had to be scrutinized by a human, it would be very tedious. LabSolutions MD can quickly and easily find optimal analytical conditions in a systematic and automated manner.
Validation Phase: Robustness Evaluation
Validation verifies that small variations in the optimized analytical condition settings affect measurement values only within an allowable range.
LabSolutions MD can create a sequential experimental design to perform robustness evaluation. Robustness evaluation is important to understand how variations in parameters will affect results and ensure the reliability of methods. LabSolutions MD creates a sequential experimental design automatically by changing the parameters of an optimized method in a small range to evaluate the robustness.
Continue reading or get more insights into what LabSolutions MD can offer your laboratory here!
Application Spotlight: Efficient Method Development on Pharmaceutical Impurities Using Single Quadrupole Mass Spectrometer
This article introduces an example of its use for the development of a robust LC method for impurities on Montelukast (a medication used in the maintenance treatment of asthma). By changing each parameter of the gradient program, the resolution of Montelukast and each impurity was evaluated by visualizing through “design space”.
By leveraging the software's capabilities, the article delves into the optimization study, where variations in gradient program parameters are systematically assessed using the design space concept. In essence, it was demonstrated how LabSolutions MD, coupled with LCMS-2050, proves superior capabilities in identifying each impurity accurately, even in the case of similar spectra. Moreover, visualizing and overlaying the patterns of resolution for each compound and RT of last eluting peak enables a more efficient (resolution) and fast (analysis time) method be easily developed regardless of user experience.
Get a copy of our application notes here to explore how LabSolutions MD eases your pharmaceutical impurity analysis.
Introducing Shimadzu's Analytical Intelligence in AQbD Method Development
To discover LabSolutions MD in detail, attend our session on Digital Classrooms to understand how LabSolutions MD automatically tests out different configurations in a systematic manner according to ICH guidelines.
Hear not only from us - but also from the customers - how the software can transform your experience in the laboratory.
Learn more about Shimadzu’s other cutting-edge technologies at our Resource Library and Digital Classrooms. In addition, stay connected with us here.


