TOC in Water: Monitoring Water Quality for Safety and Quality For Pharmaceutical Uses

TOC in Water: Monitoring Water Quality for For Pharmaceutical Uses
A total organic carbon (TOC) analyzer is an analytical instrument that measures the total amount of organic carbon contained in water. It is used for quality control of public drinking water, control and evaluation of pharmaceutical manufacturing processes, and a wide variety of other applications.
Importance of Measuring TOC in the Pharmaceutical Industry

Pharmaceutical water used in drug manufacturing processes must have a high level of safety and minimal impurities. Total organic carbon (TOC) is used in control of organic impurities in pharmaceutical water, and control standards corresponding to the application are provided in the pharmacopeias used in various countries. For example, the Japanese Pharmacopoeia (JP) sets a limit of "not more than 0.500 mg/L" for TOC in purified water and water for injection (bulk). Quick and easy control of organic impurities is possible with Shimadzu TOC analyzers, even in cases where measurement with this high sensitivity/high accuracy is required.
Click below to learn more about TOC System For Pharmaceutical Production, or read on for the fundamentals of TOC!
TOC Measurement Methods

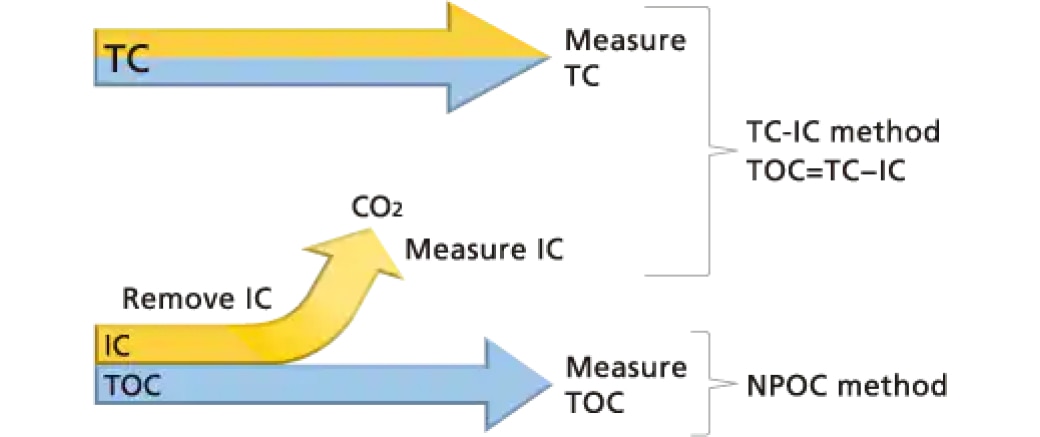
The following two methods are used for determining TOC present in water.
TC-IC Method:
TOC is determined as the difference between TC (Total Carbon) and IC (Inorganic Carbon) measurement values. (TOC = TC - IC)
NPOC Method:
TOC is determined by measuring TC in samples pretreated to remove IC. (TOC = TC)
Measuring IC
For TOC measurement, IC refers to the total quantity of inorganic carbon contained in (where CO2 indicates dissolved carbon dioxide, HCO3- bicarbonate ions, and CO32- carbonate ions). The quantities of dissolved carbon dioxide, bicarbonate ions, and carbonate ions in water are kept in an equilibrium that depends on the pH level of the water, according to the expression below.

If pH decreases, the equilibrium moves to the left in the expression above. At a pH of 3 or lower, almost all IC becomes dissolved carbon dioxide.
Based on that principle, IC is measured by adding acid to lower the sample pH below 3 and then measuring the CO2 extracted from the sample by bubbling in a CO2-free gas atmosphere.
Using TC-IC and NPOC Methods
Both the TC-IC method and the NPOC method are for measuring TOC, but which of the measurement methods to use is determined based on sample characteristics.
For samples with low IC concentration levels, such as public drinking water or purified water, the NPOC method is used because the TC-IC method is prone to measurement error that could result in lower measurement accuracy.
On the other hand, for samples with large amounts of volatile organic compounds or samples that are prone to foaming, for example, the TC-IC method is used because the NPOC method can result in loss of volatile organic compounds from samples during the bubbling in a CO2-free gas pretreatment step or the NPOC method can prevent accurate sample quantity measurements due to foaming.
Measurement Principle: Oxidation Methods
TOC analyzers generally decompose organic substances in a sample by oxidation and measure the generated carbon dioxide. Methods of oxidative decomposition are broadly divided into the combustion catalytic oxidation method and the wet chemical oxidation method.
Combustion Oxidation Method
One of the main features of this method is its ability to efficiently oxidize organic carbon matter that is otherwise resistant to decomposition, such as carbon matter that contains insoluble or macromolecular organic substances.
The sample is injected into a high-temperature (650 to 1,200°C) combustion furnace to incinerate all organic carbon in the sample and measure it as fully oxidized carbon dioxide.
Because samples are combusted at a high temperature, the method enables complete oxidative dissociation of carbon even in persistent organic matter, or carbon in suspended substances or other water-insoluble particulate organic matter.
Due to the simplicity of using heat/combustion as the principle for oxidation, the method requires no reagents for pretreatment or post-treatment processes.
Wet Oxidation Method
With this method, an oxidizing agent is added to samples to chemically decompose carbon in organic matter for measurement as carbon dioxide. Though heat (up to 100°C) or ultraviolet irradiation can be applied to promote the oxidation reaction, the ability of the chemical reaction to oxidatively decompose matter is weaker than combustive oxidation, which tends to result in lower carbon recovery rates from suspended or other particulate organic matter, or persistent substances.
Therefore, due to its superior oxidative reaction, the combustion oxidation method is commonly used to measure TOC levels in environmental water, factory effluent, and other such samples, where water samples often contain large amounts of insoluble organic carbon.
Generally, combustion catalytic oxidation has high oxidizability and enables oxidative decomposition regardless of the type and state of existence of organic substances. For this reason, it is the optimum method for measurements of environmental water and wastewater, but since this method also realizes high sensitivity, with a limit of detection of 4 μg/L, it amply satisfies the requirements for measurement of pure water. The wet chemical oxidation method provides higher sensitivity measurement performance and is the optimum method for measurements of ultrapure water. The table below provides a summary:
| Wet Chemical Oxidation | Combustion Catalytic Oxidation | |
|---|---|---|
| Features | High sensitivity • Optimum for measurement of ultrapure water • Limit of detection < 1μg/L |
High oxidizability • Enables oxidative decomposition regardless of type and state of existence of organic substances • Limit of detection < 4 μg/L |
| Main applications | Ultrapure water, pure water (pharmaceutical water), tap water | Pure water (pharmaceutical water), tap water, environmental water, waste water |
Introducing Shimadzu's TOC-V/L Series For Pharmaceutical Production
Currently, there is a strong demand for the introduction of development, manufacturing, and control systems that adhere to GxP guidelines within pharmaceutical facilities. The significance of water quality control, aligned with GxP regulations, is particularly crucial in the pharmaceutical industry, where water serves not only as a raw material but also as rinsing water.
To address this demand, Shimadzu's TOC-V/L series presents as an optimal solution that aligns not only with USP (United States Pharmacopeia) and JP (Japanese Pharmacopeia) but also with other world standards (EPA, ASTM, JIS, ISO, etc.), while demonstrating its outstanding performance in TOC control and cleaning validation of pharmaceutical water.


