Observation Examples of Metals
Observation of Copper Plate
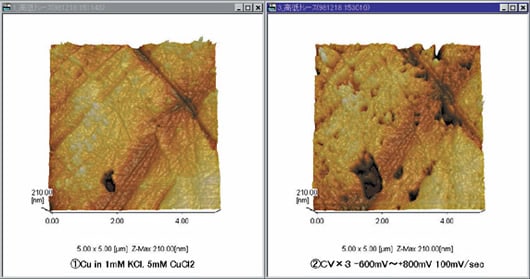
An electrochemical reaction has been caused in a copper plate in solution, and the surface form changes are observed in situ. This is the stage where cyclic voltammetry from - 600 mV to 800 mV has been repeated three times in a solution of 1 mM KCL, 5 mM CuCl2. (Using electrochemical solution cell and electrochemical controller)


